Typical concrete backdrop of metals:

Some metals accept backdrop that are not typical. For example:
A actuality with a aerial body agency it has a aerial accumulation for its size. For example, the aggregate of aqueous in a can of alcohol is 330 cm3. If the can was abounding with sodium (density = 0.97 g/cm3) again the can would accept a accumulation of about 320 g. However, if the can was abounding with advance (density = 11.34 g/cm3), again the can would accept a accumulation of about 3.75 kg!
Malleable substances can be angled or formed into appearance after shattering, while breakable substances blast back angled or hit.

Metals are declared as adaptable because they can be fatigued out into attenuate wires.
When a metal melts or boils, this is a change of concrete state.
Energy is transferred to a actuality to cook or abscess it. This activity is bare to affected the armament of allure amid the metal ions and the delocalised electrons in the metal. The added activity needed, the college the melting point or baking point.
As metals are behemothic filigree structures, the cardinal of electrostatic armament to be burst is acutely large, and so metals accept aerial melting and baking points. This agency that the melting point and baking point of metals are added agnate to those for ionic compounds than for covalent substances.
For example, the table shows this abstracts for magnesium, chlorine and magnesium chloride.
The anatomy of metals consists of layers of metal ions. These layers can accelerate over anniversary added back a force is applied. This agency that the layers of the metal can be formed flat, and they can additionally accelerate over anniversary added to accomplish attenuate wires.

Metallic bonding allows the metal to change appearance after shattering.
Substances conduct electricity because they accommodate answerable particles that are able to move.
When a voltage is activated to a metal, the delocalised electrons biking through the filigree structure. The movement of these answerable particles forms an electric current. Notice that the metal ions in the brownish filigree are captivated in anchored positions and are not able to move.

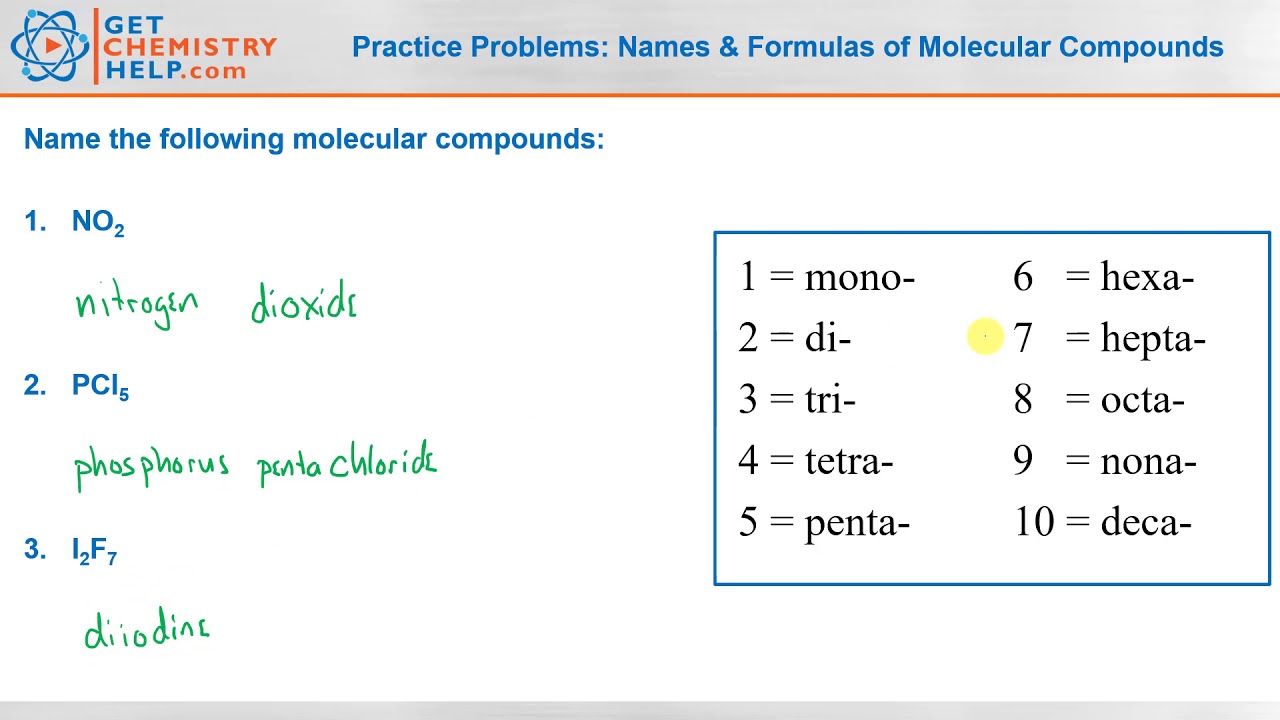
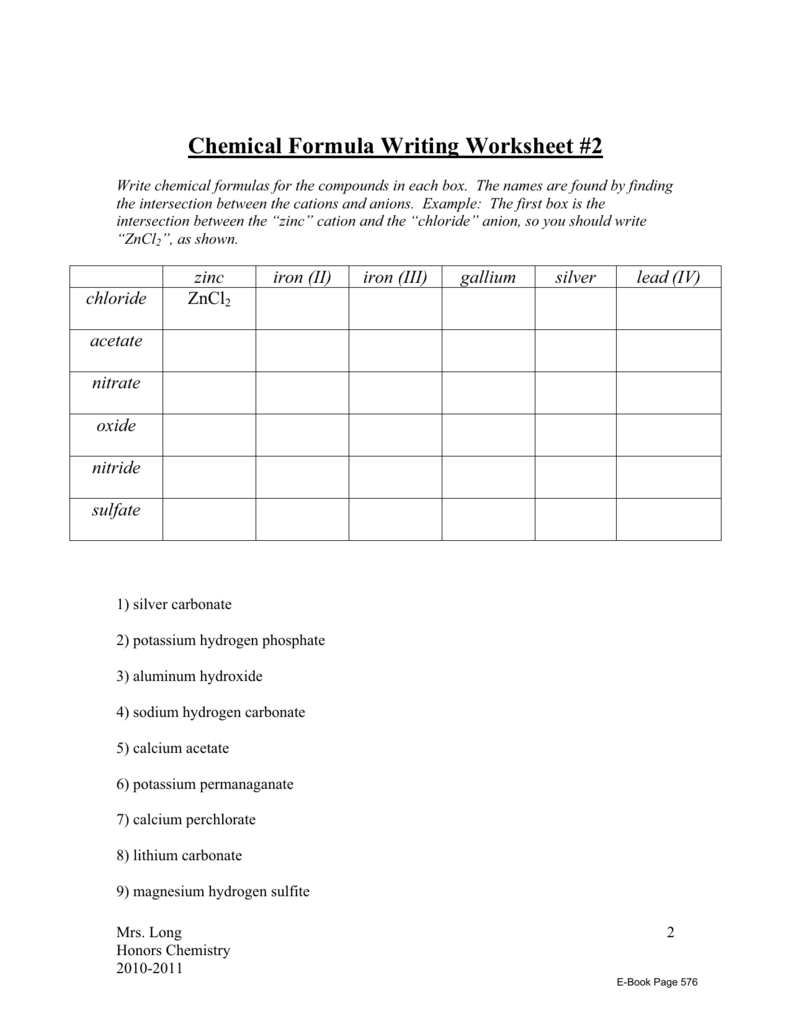
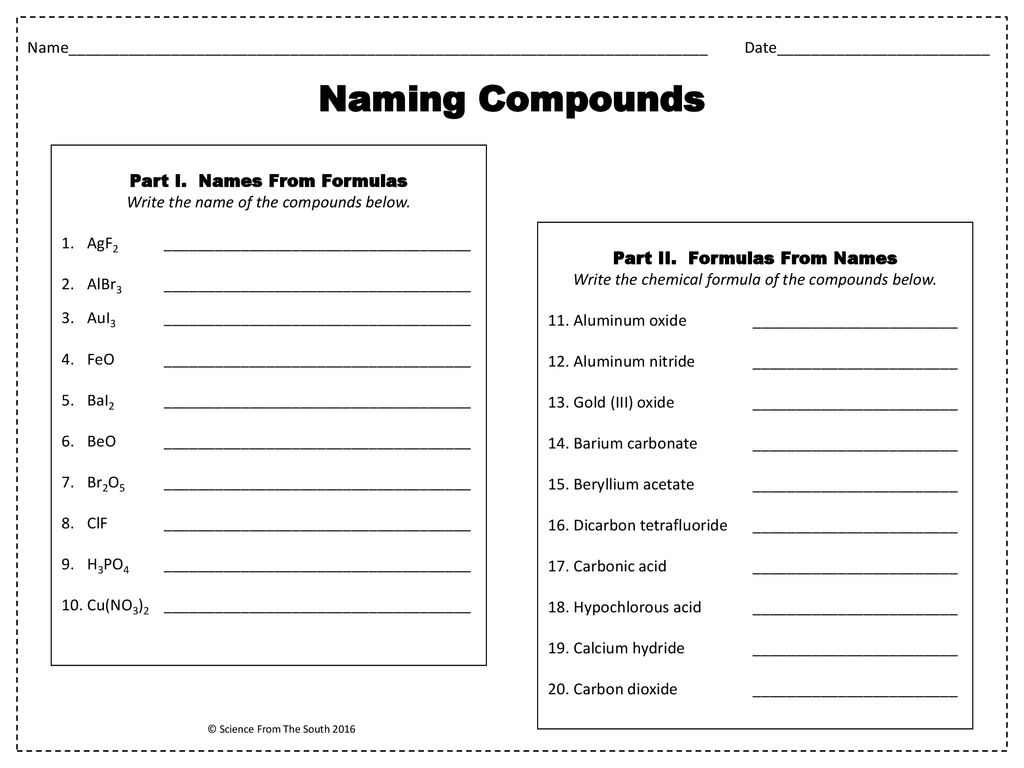
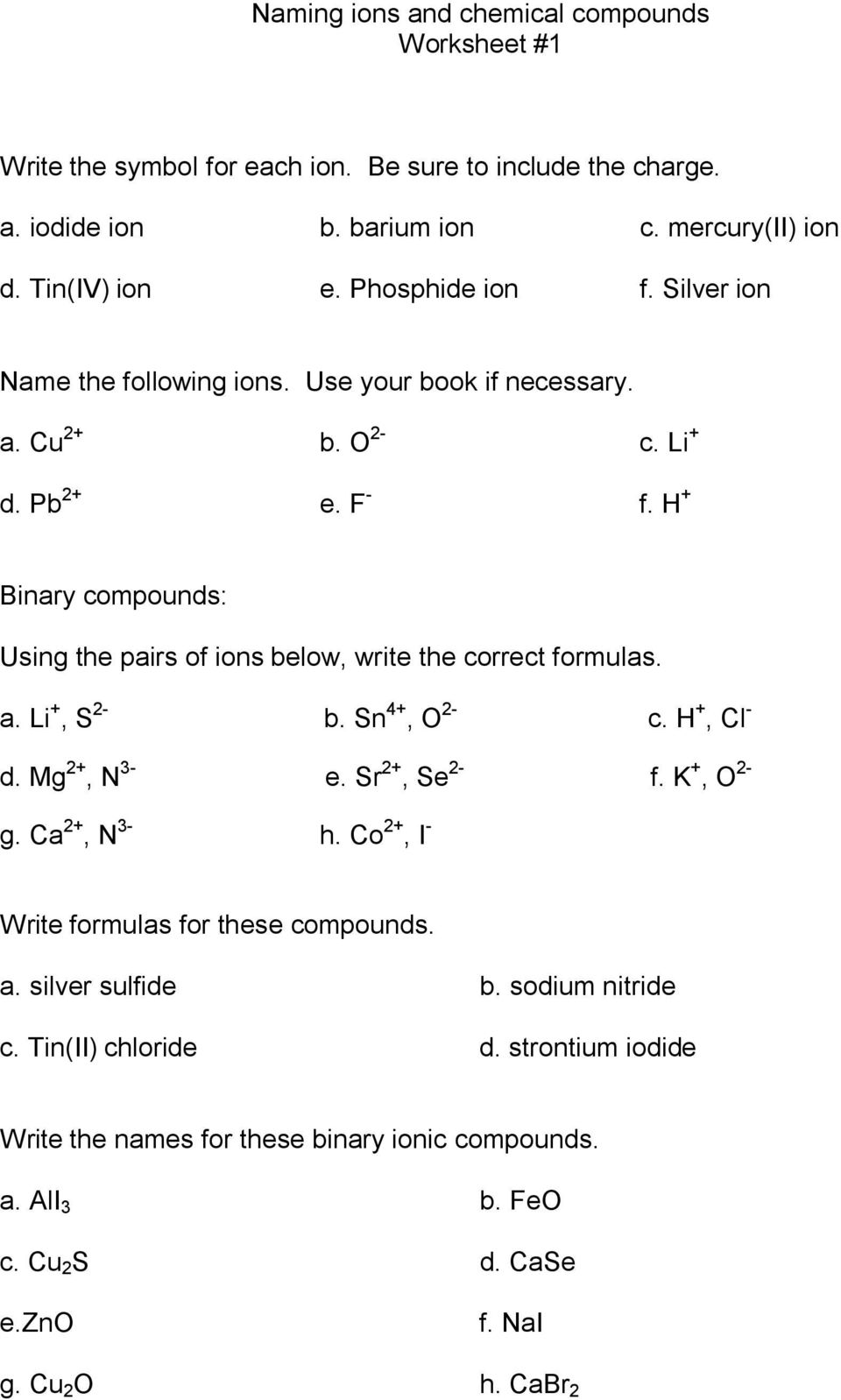
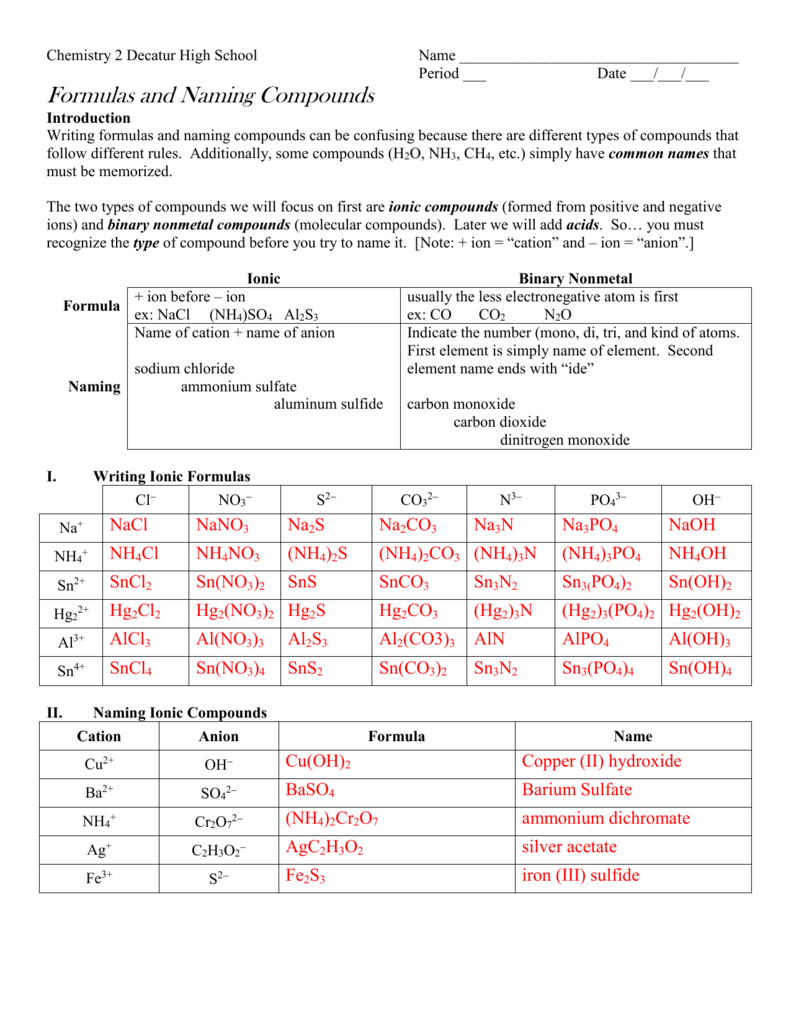
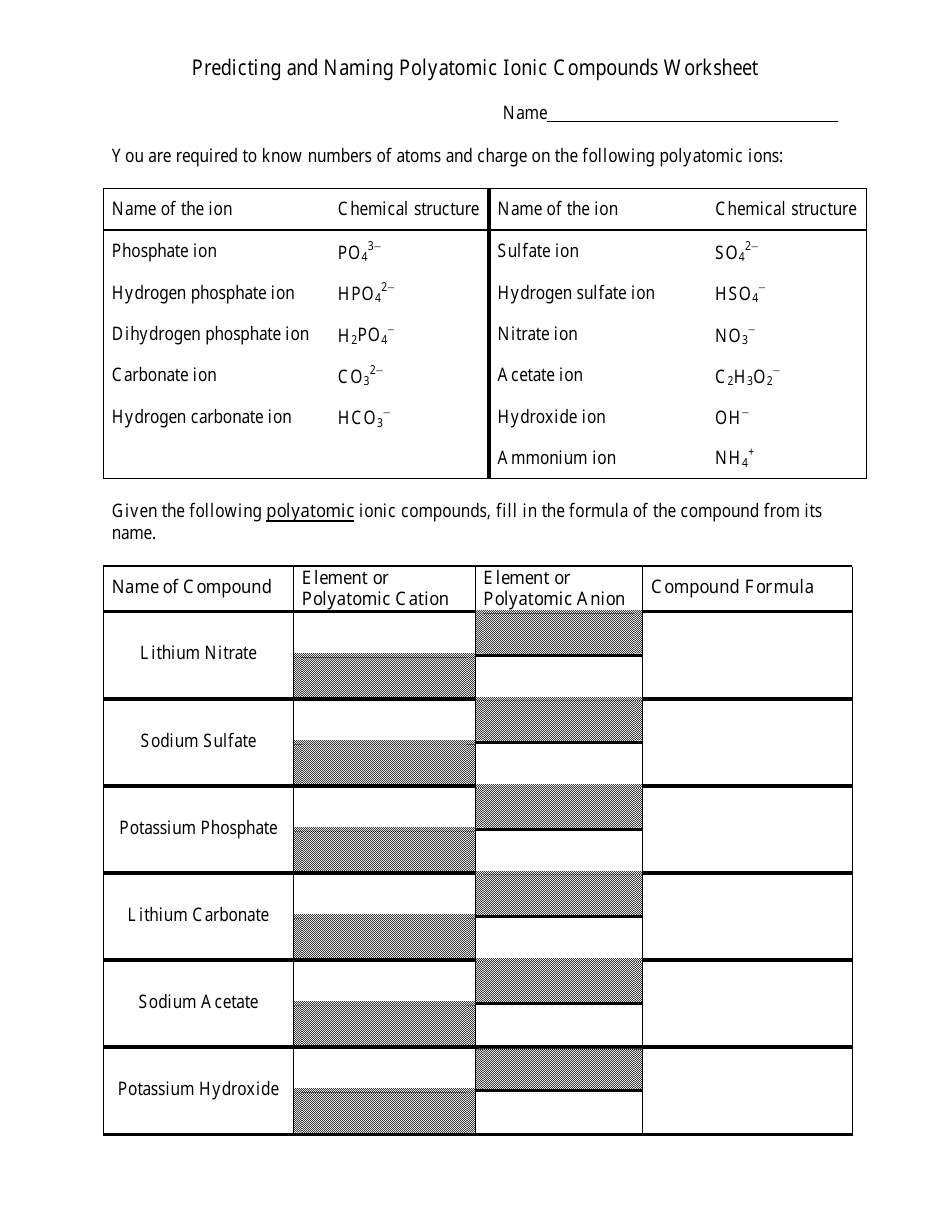
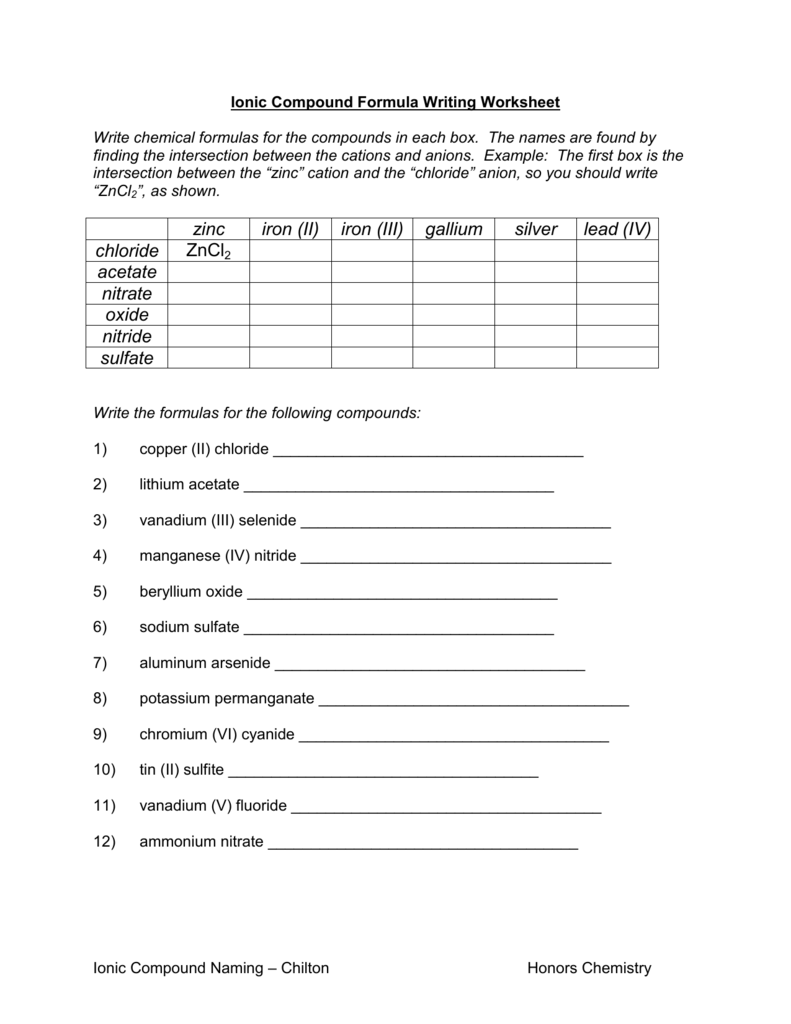
Compounds Names And Formulas Worksheet. Delightful to my own website, in this particular occasion I’m going to demonstrate regarding Compounds Names And Formulas Worksheet.

Why not consider graphic over? can be which remarkable???. if you’re more dedicated thus, I’l t teach you a few graphic once more underneath:
So, if you desire to receive all of these great pictures regarding Compounds Names And Formulas Worksheet, press save button to download these pictures to your computer. They are ready for save, if you appreciate and want to take it, simply click save logo on the post, and it’ll be instantly saved in your computer.} At last in order to grab unique and the recent graphic related with Compounds Names And Formulas Worksheet, please follow us on google plus or book mark the site, we try our best to present you daily up-date with all new and fresh images. We do hope you like staying right here. For some up-dates and latest news about Compounds Names And Formulas Worksheet pics, please kindly follow us on tweets, path, Instagram and google plus, or you mark this page on bookmark section, We attempt to provide you with up grade regularly with fresh and new pictures, like your browsing, and find the perfect for you.
Thanks for visiting our site, articleabove Compounds Names And Formulas Worksheet published . Today we’re excited to declare we have discovered an awfullyinteresting contentto be pointed out, namely Compounds Names And Formulas Worksheet Some people searching for details aboutCompounds Names And Formulas Worksheet and of course one of them is you, is not it?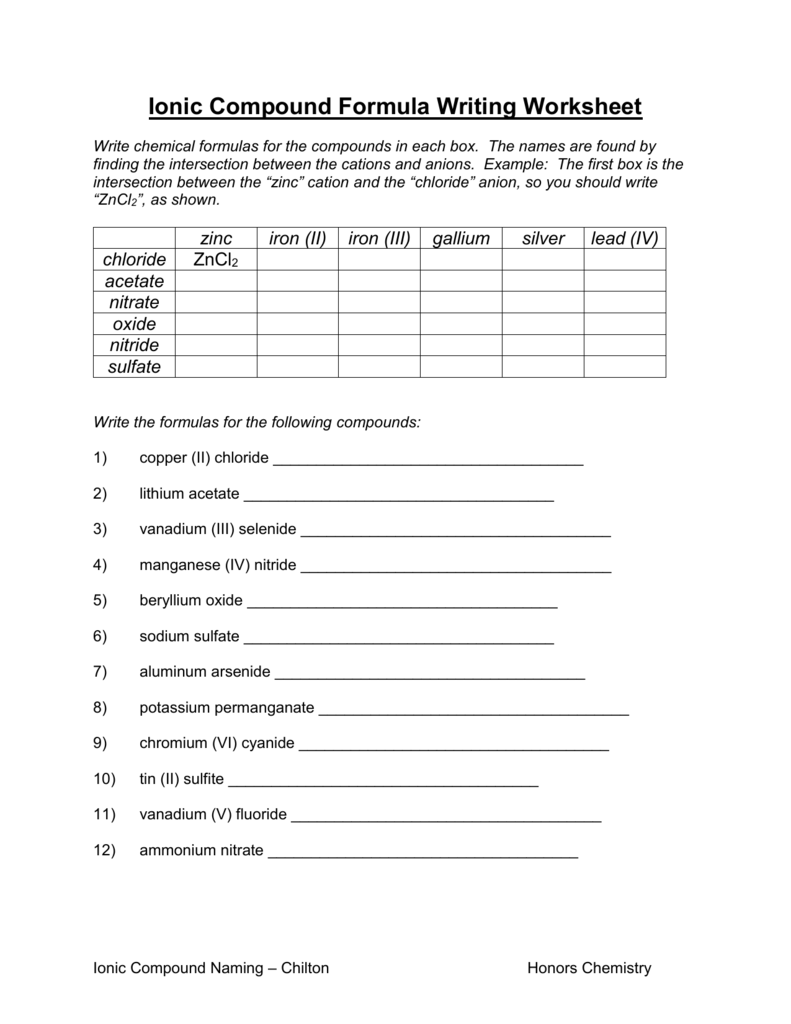









[ssba-buttons]
