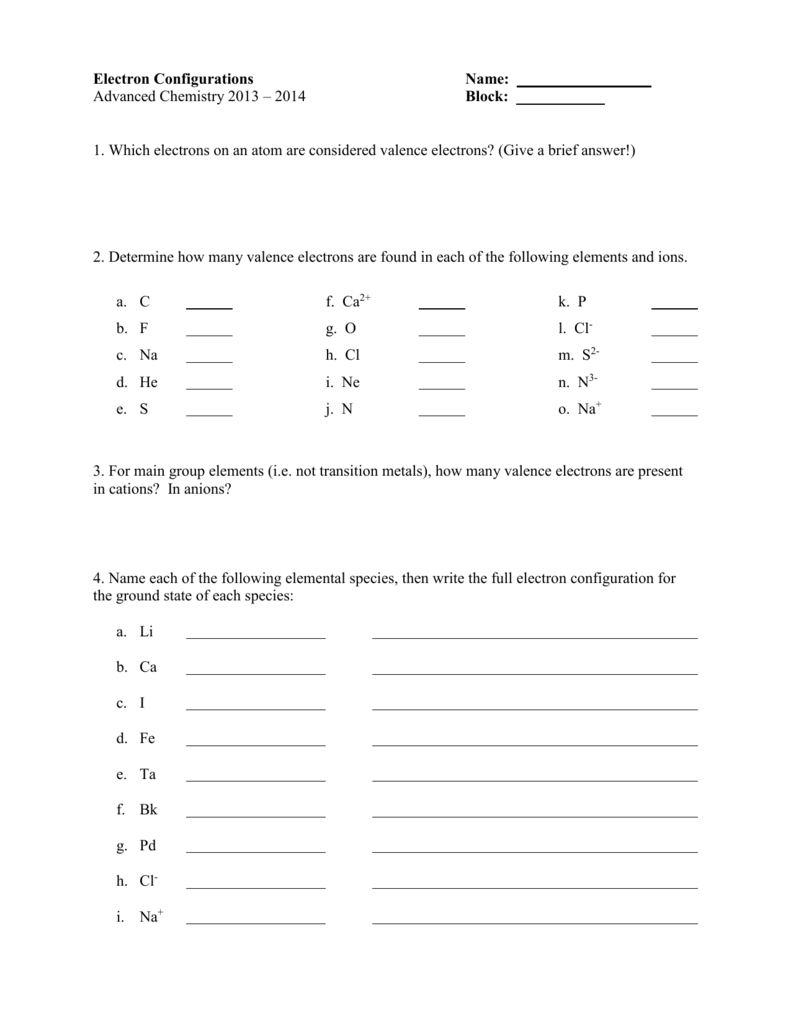
Electron Configuration Practice Worksheet. There is a simple pattern that you will see in a few minutes through the use of the beneath examples. Generally, it goes to be the case that electrons reside in pairs and spin in opposite instructions. 3) Trace out the variety of electrons present within the outer most shell. However, Hund’s rule strictly follows the speculation of atomic spectra.
Below is the potential for variety of valence electrons of transition metals based on group number. 2) Choose any component of your selection from the periodic table. This quantum number is in any other case popular as orbital quantum quantity.

To make it simple and convenience to write down, we will write the electronic configuration of Aluminium utilizing noble gas notation as 3s2 3p1. 3) In case of cation, subtract the electrons across the component from the entire number of valence electrons whereas drawing the dot diagram. Based on this info, allow us to study ground and excited state ranges and in addition in regards to the variations between these two states of power levels.
Electron Configuration Worksheet
There is an easy pattern that you will see in a couple of minutes by utilizing the beneath examples. It’s finest to be taught the topic of electron configurations by instance, as it might easily take 1,000,000 phrases to describe.
Patterns between valence electrons, energy levels, and orbitals are explored. Electron configurations embody parts as much as the 7th vitality degree. This worksheet supplies 10 examples for college kids to apply writing electron configurations, orbital notations, and noble gasoline notations for various components.
Electron Configuration Elements 1
Put the arrow within the lowest box, similar to the lowest energy, meaning it is the closest to the nucleus. Writing digital configurations for the weather present within the initial intervals and teams of the periodic desk is straightforward and simple.
The electron configuration of a component is a normal representation of its electron association in the orbitals of its atom. This notation additionally helps in understanding the bonding capacity of electrons in an atom through magnetic and different chemical options.
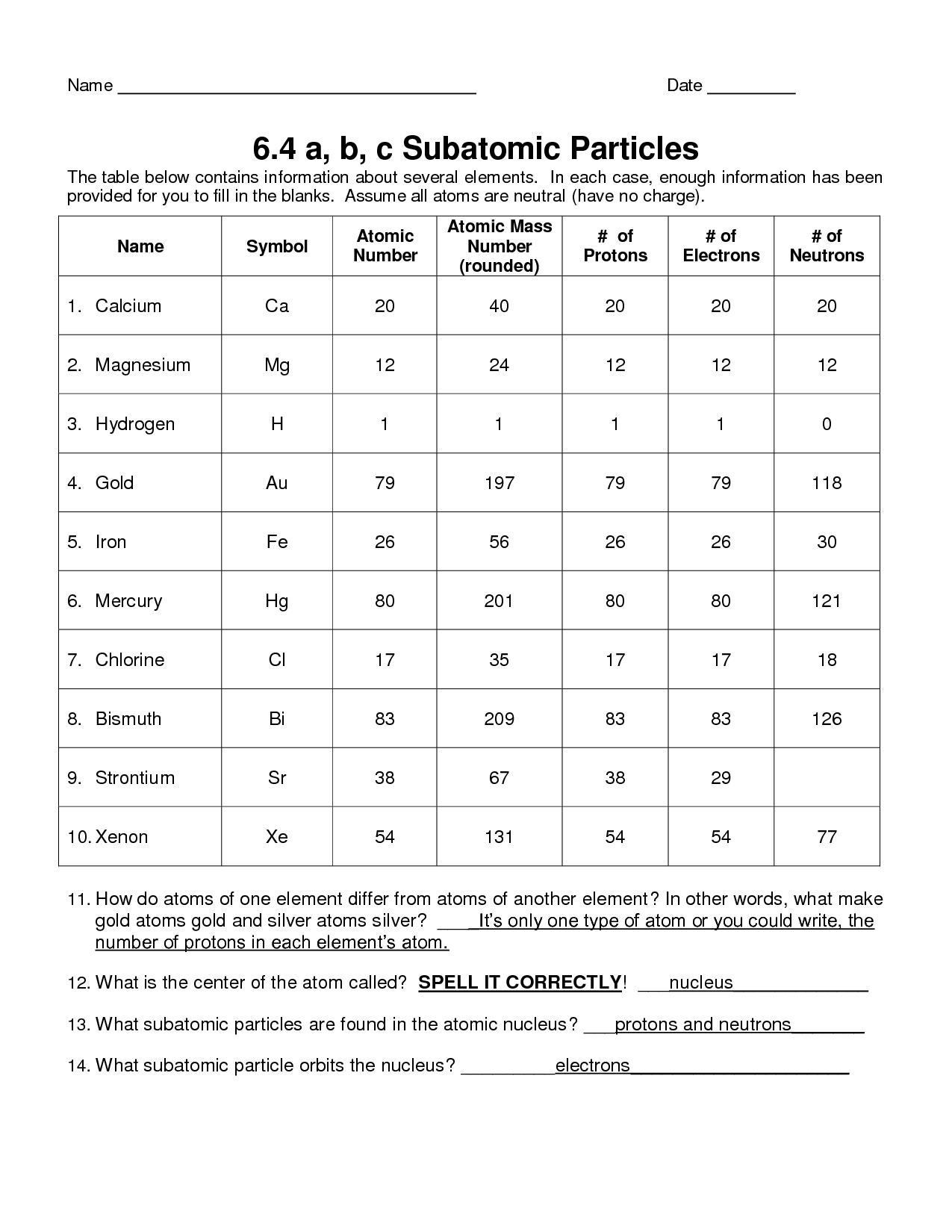
Read the labels of several commercial merchandise and determine monatomic ions of at least 4 transition parts contained within the products. Write the whole electron configurations of these cations.
Well, positively charged electrons get attracted by negatively charged electrons while probably charged electrons repel each other.
Motion Graphs Worksheet Reply Key Elegant Projectile Motion Worksheet
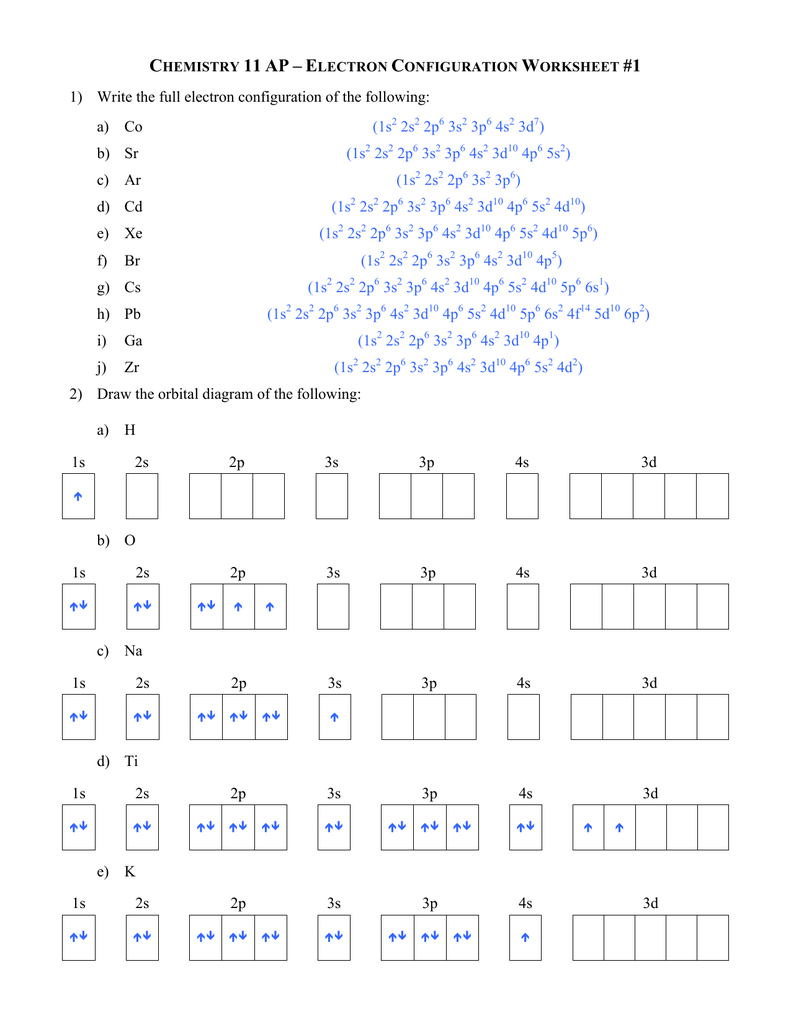
There stays 1 unpaired electron for Cu in the 3d orbtial. Finally, the straightforward approach to learn to discover an electron configuration, also referred to as an orbital diagram or the quantum numbers.

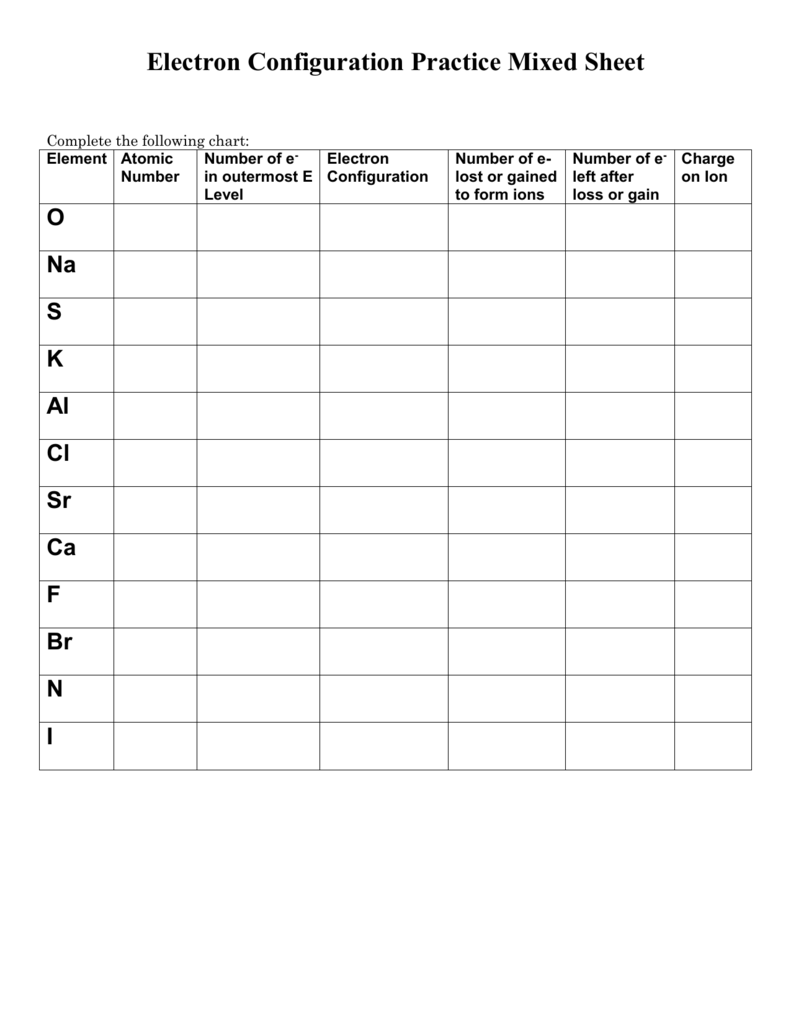
2) In case of anion molecule, add the additional electrons across the component while drawing dot diagram. The variety of dots around the factor symbolize the number of valence electrons of that specific element. 2) Using Octet Rule, prepare the electrons to its orbital shells based on electron configuration.
At this point, we all are aware of that an electron’s location is unsure and solely reveal their probability of exact location around the nucleus. The ‘ℓ’ values remains between zero and n-1 whereas relying on the values of principal quantum quantity. Here, if the n value is 2, then the ‘ℓ’ value is either zero or 1.

This on-line quiz is intended to offer you additional follow in writing electron configurations for every of the first 102 chemical elements. Read the labels of a quantity of commercial products and identify monatomic ions of no less than six main group parts contained in the merchandise. Write the whole electron configurations of those cations and anions.
And additionally said that these atomic orbitals encompasses of electrons at highest risk. The 1s orbital is full, the 2s orbital is full, and there are 4 electrons to draw in the three packing containers in the 2p orbital. As per Hund’s Rule, there could be three arrows pointing up in the 2p orbital and 1 pointing down.

Write the complete electron configuration for every isotope. Copper Cu is factor 29 with 29 electrons when it’s neutral. Compared to iron in the previous example, there are an additional three electrons to pair up as downward pointing arrows within the 3d orbital.

The electrons which don’t participate in any kind of chemical bonding and don’t discuss with valence electrons are core electrons. These electrons are usually present in inner power levels and totally occupied and therefore referred to chemically inert electrons. So with the help of orbital diagram, we will easily find out which type of atomic orbitals crammed out and which are partially occupied with electrons.

Barium is a extremely reactive alkaline earth metallic with atomic number fifty six and bears the image ‘Ba’. Since it is highly reactive, we can’t find this metal in its free state and all the time remains in combination with other metals.

According to Pauli Exclusion Principle, two or extra electrons of a single atom cannot occupy the identical quantum state and possess the identical quantum values. As all of us already know, electrons bear charge i.e. either adverse or positive, and are free to vary their areas often.

1) Pick a periodic table the place you will discover all the variety of elements in the boxes. And learn about periodic table terminology like rows, columns, intervals and teams. Hund’s rule denotes that electrons must occupy each single orbital of a subshell with at least one electron with identical spin direction.

Let us be taught extra concerning the digital configuration along with some superior worksheets and orbital diagrams on this article. Argon Ar is component 18 with 18 electrons when it’s impartial.

Therefore, we are in a position to say that the transcribed description of orbital diagram is nothing however electron configuration. Consider Bromine element positioned within the Group VII, Period four of the periodic table. It has 35 electrons and among which 7 electrons are valence electrons.
6) Check out for each atom whether or not it possess octet configuration. If any atom does not have octet configuration, then you have to fulfil the octet valence of each particular person atom. 3) Trace out the variety of electrons current in the outer most shell.

As per Hund’s Rule, all three arrows level up within the 2p orbital. After putting 2 arrows in the first field referred to as the 1s orbital and one other 2 arrows within the second field referred to as the 2s, there are nonetheless 2 extra electrons to attract. Lithium Li is element three with three electrons when it’s neutral.
The digit on the ones place of the group number refers again to the variety of valence electrons of a component. To put it simply, each individual electron encompasses of 4 quantum numbers and two electrons should exhibit reverse spins when located in the identical orbital.

Thallium was used as a poison within the Agatha Christie mystery story “The Pale Horse.” Thallium has two potential cationic forms, +1 and +3. Write the electron structure of the +1 cation of thallium. 5) Then, allot the lone pair of electrons to every single atom of a molecule.
And while changing the noble gas element is written in square brackets. In this fashion, abbreviated electron configuration is far more helpful for components that has larger atmic numbers. Electron dot configuration is a sort of diagrammatic illustration of number of valence electrons of an element within the form of dots around the component.

Transition metals does not have traditional valence electrons. Hence, we cannot predict the number of valence electrons of a transition steel with sure number.

To determine the electronic configuration of an element, one should observe three necessary principles from quantum mechanics. These 4 atomic orbitals are present around the nucleus of an atom and symbolize different energy states. We now place the remaining 6 electrons in the 3d orbital, as per Hund’s Rule.

Hydrogen is factor 1 on the periodic desk with 1 electron when it’s impartial. The concept is to draw an arrow for each electron, so on this case we simply have one arrow to draw.
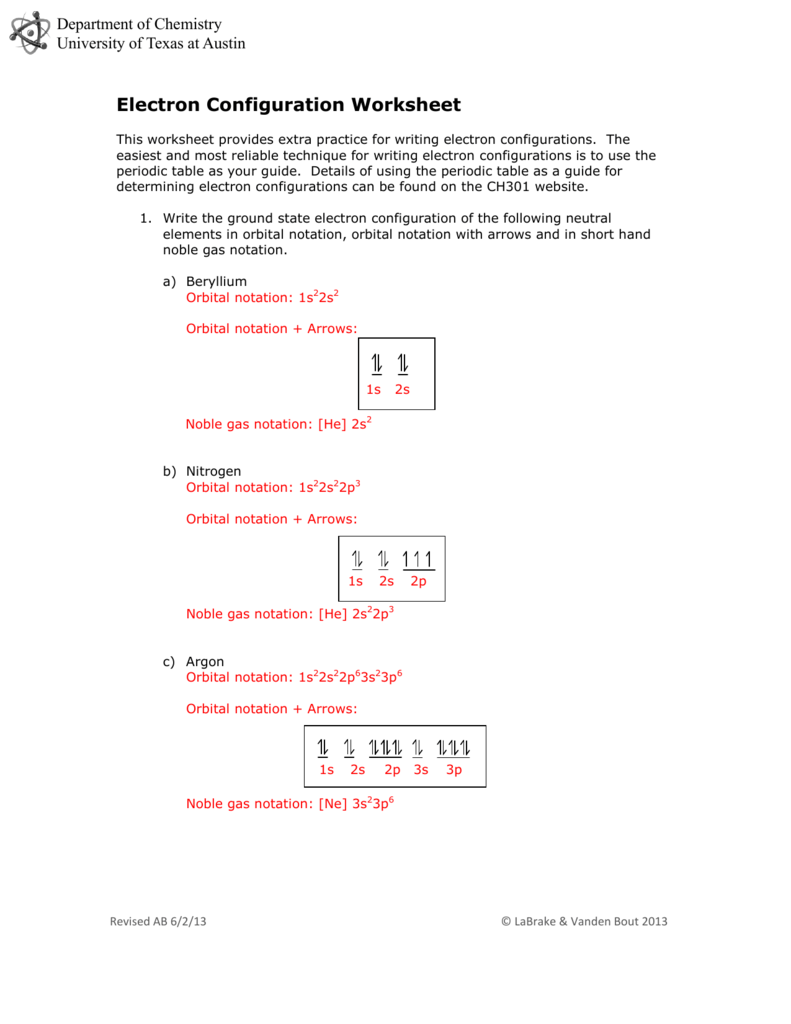
Students must first determine the element in each instance by reading the �clue� that describes the element�s identity. This worksheet can be used in any Chemistry class, whatever the students� ability stage.

And the ‘n’ worth is decided based mostly on the space of power level from the nucleus of the atom. These values vary begin from 1 to n…, whereas n denotes the value of the outermost shell occupied with electron.
[ssba-buttons]