Takai, Y. & Nakanishi, H. Nectin and afadin: atypical organizers of intercellular junctions. J. Corpuscle Sci. 116, 17–27 (2003).

Takai, Y., Irie, K., Shimizu, K., Sakisaka, T. & Ikeda, W. Nectins and nectin-like molecules: roles in corpuscle adhesion, migration, and polarization. Blight Sci. 94, 655–667 (2003).
Geraghty, R. J., Krummenacher, C., Cohen, G. H., Eisenberg, R. J. & Spear, P. G. Access of αherpesviruses advised by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280, 1618–1620 (1998).
Warner, M. S. et al. A corpuscle apparent protein with herpesvirus access action (HveB) confers susceptibility to infection by mutants of canker canker virus blazon 1, canker canker virus blazon 2, and pseudorabies virus. Virology 246, 179–189 (1998).
Takahashi, K. et al. Nectin/PRR: an immunoglobulin-like corpuscle adherence atom recruited to cadherin-based adherens junctions through alternation with afadin, a PDZ domain-containing protein. J. Corpuscle Biol. 145, 539–549 (1999). Identifies nectin as a CAM that anon binds to afadin with Ca2 -independent corpuscle adherence action and that colocalizes with cadherin at AJs.
Kakunaga, S. et al. Nectin-like molecule-1/TSLL1/SynCAM3: a neural tissue-specific immunoglobulin-like cell–cell adherence atom localizing at non-junctional acquaintance sites of presynaptic assumption terminals, axons, and glia corpuscle processes. J. Corpuscle Sci. 118, 1267–1277 (2005).
Maurel, P. et al. Nectin-like proteins arbitrate axon Schwann corpuscle interactions forth the internode and are capital for myelination. J. Corpuscle Biol. 178, 861–874 (2007).
Spiegel, I. et al. A axial role for Necl4 (SynCAM4) in Schwann cell–axon alternation and myelination. Nature Neurosci. 10, 861–869 (2007).
Kuramochi, M. et al. TSLC1 is a tumor-suppressor gene in animal non-small-cell lung cancer. Nature Genet. 27, 427–430 (2001).
Fujito, T. et al. Inhibition of corpuscle movement and admeasurement by cell–cell contact-induced alternation of Necl-5 with nectin-3. J. Corpuscle Biol. 171, 165–173 (2005). Proves that the downregulation of NECL-5, which is accomplished by its auto -interaction with nectin-3 afterward cell–cell acquaintance is one of the mechanisms that underlie acquaintance inhibition of corpuscle movement and proliferation.
Mendelsohn, C. L., Wimmer, E. & Racaniello, V. R. Cellular receptor for poliovirus: atomic cloning, nucleotide sequence, and announcement of a new affiliate of the immunoglobulin superfamily. Corpuscle 56, 855–865 (1989). Shows the cDNA arrangement and announcement arrangement of PVR in bodies and analyses its atomic characteristics to appraise the approach of poliovirus adapter and replication.
Koike, S. et al. The poliovirus receptor protein is produced both as membrane-bound and buried forms. EMBO J. 9, 3217–3224 (1990).
Chadeneau, C., LeCabellec, M., LeMoullac, B., Meflah, K. & Denis, M. G. Over-expression of a atypical affiliate of the immunoglobulin superfamily in Min abrasion abdominal adenomas. Int. J. Blight 68, 817–821 (1996).
Chadeneau, C., LeMoullac, B. & Denis, M. G. A atypical affiliate of the immunoglobulin gene superfamily bidding in rat blight corpuscle lines. J. Biol. Chem. 269, 15601–15605 (1994).
Lim, Y. P., Fowler, L. C., Hixson, D. C., Wehbe, T. & Thompson, N. L. TuAg.1 is the alarmist isoform of the rat colon tumor-associated antigen pE4 and a affiliate of the immunoglobulin-like supergene family. Blight Res. 56, 3934–3940 (1996).
Erickson, B. M., Thompson, N. L. & Hixson, D. C. Tightly adapted consecration of the adherence atom necl-5/CD155 during rat alarmist about-face and astute alarmist injury. Hepatology 43, 325–334 (2006).
Hirota, T., Irie, K., Okamoto, R., Ikeda, W. & Takai, Y. Transcriptional activation of the abrasion Necl-5/Tage4/PVR/CD155 gene by fibroblast advance agency or oncogenic Ras through the Raf–MEK–ERK–AP-1 pathway. Oncogene 24, 2229–2235 (2005).
Kakunaga, S. et al. Enhancement of serum- and platelet-derived advance factor-induced corpuscle admeasurement by Necl-5/Tage4/poliovirus receptor/CD155 through the Ras–Raf–MEK–ERK signaling. J. Biol. Chem. 279, 36419–36425 (2004).
Fisher, H. W. & Yeh, J. Acquaintance inhibition in antecedents formation. Science 155, 581–582 (1967).
Bell, P. B. Jr. Acquaintance inhibition of movements in adapted and nontransformed cells. Birth Defects Orig. Artic. Ser. 14, 177–194 (1978).
Yagi, T. & Takeichi, M. Cadherin superfamily genes: functions, genomic organization, and neurologic diversity. Genes Dev. 14, 1169–1180 (2000).
Takeichi, M. The cadherin superfamily in neuronal access and interactions. Nature Rev. Neurosci. 8, 11–20 (2007).
van der Flier, A. & Sonnenberg, A. Function and interactions of integrins. Corpuscle Tissue Res. 305, 285–298 (2001).
Comoglio, P. M., Boccaccio, C. & Trusolino, L. Interactions amid advance agency receptors and adherence molecules: breaking the rules. Curr. Opin. Corpuscle Biol. 15, 565–571 (2003).
Perez-Moreno, M., Jamora, C. & Fuchs, E. Sticky business: orchestrating cellular signals at adherens junctions. Corpuscle 112, 535–548 (2003).
Yap, A. S. & Kovacs, E. M. Direct cadherin-activated corpuscle signaling: a appearance from the claret membrane. J. Corpuscle Biol. 160, 11–16 (2003).
Mueller, S., Cao, X., Welker, R. & Wimmer, E. Alternation of the poliovirus receptor CD155 with the dynein ablaze alternation Tctex-1 and its affiliation for poliovirus pathogenesis. J. Biol. Chem. 277, 7897–7904 (2002).
Takekuni, K. et al. Direct bounden of corpuscle polarity protein PAR-3 to cell–cell adherence atom nectin at neuroepithelial beef of developing mouse. J. Biol. Chem. 278, 5497–5500 (2003).

Yageta, M. et al. Direct affiliation of TSLC1 and DAL-1, two audible bump suppressor proteins in lung cancer. Blight Res. 62, 5129–5133 (2002).
Shingai, T. et al. Implications of nectin-like molecule-2/IGSF4/RA175/SgIGSF/TSLC1/SynCAM1 in cell–cell adherence and transmembrane protein localization in epithelial cells. J. Biol. Chem. 278, 35421–35427 (2003).
Mandai, K. et al. Afadin: a atypical actin filament-binding protein with one PDZ area localized at cadherin-based cell-to-cell adherens junction. J. Corpuscle Biol. 139, 517–528 (1997). Shows that afadin antiseptic from rat academician has F-actin-binding backdrop and accurately localizes at cadherin-based AJs, advertence a role for afadin in bond AJ structures to the actin cytoskeleton.
Prasad, R. et al. Cloning of the ALL-1 admixture partner, the AF-6 gene, circuitous in astute myeloid leukemias with the t(6;11) chromosome translocation. Blight Res. 53, 5624–5628 (1993).
Saito, S. et al. Complete genomic anatomy DNA polymorphisms, and another splicing of the animal AF-6 gene. DNA Res. 5, 115–120 (1998).
Satoh-Horikawa, K. et al. Nectin-3, a new affiliate of immunoglobulin-like corpuscle adherence molecules that shows homophilic and heterophilic cell–cell adherence activities. J. Biol. Chem. 275, 10291–10299 (2000).
Reymond, N. et al. Nectin4/PRR4, a new afadin-associated affiliate of the nectin ancestors that trans-interacts with nectin1/PRR1 through V area interaction. J. Biol. Chem. 276, 43205–43215 (2001).
Aoki, J. et al. Abrasion homolog of poliovirus receptor-related gene 2 product, mPRR2, mediates homophilic corpuscle aggregation. Exp. Corpuscle Res. 235, 374–384 (1997).
Ikeda, W. et al. Tage4/nectin-like molecule-5 heterophilically trans-interacts with corpuscle adherence atom nectin-3 and enhances corpuscle migration. J. Biol. Chem. 278, 28167–28172 (2003).
Bottino, C. et al. Identification of PVR (CD155) and Nectin-2 (CD112) as corpuscle apparent ligands for the animal DNAM-1 (CD226) activating molecule. J. Exp. Med. 198, 557–567 (2003).
Fuchs, A., Cella, M., Giurisato, E., Shaw, A. S. & Colonna, M. Cutting edge: CD96 (tactile) promotes NK cell–target corpuscle adherence by interacting with the poliovirus receptor (CD155). J. Immunol. 172, 3994–3998 (2004).
Boles, K. S., Barchet, W., Diacovo, T., Cella, M. & Colonna, M. The bump suppressor TSLC1/NECL-2 triggers NK-cell and CD8 T-cell responses through the cell-surface receptor CRTAM. Blood 106, 779–786 (2005).
Koch, A. W., Pokutta, S., Lustig, A. & Engel, J. Calcium bounden and homoassociation of E-cadherin domains. Biochemistry 36, 7697–7705 (1997).
Honda, T. et al. Antagonistic and agonistic furnishings of an extracellular fragment of nectin on accumulation of E-cadherin-based cell–cell adhesion. Genes Beef 8, 51–63 (2003).
Anastasiadis, P. Z. & Reynolds, A. B. The p120 catenin family: circuitous roles in adhesion, signaling and cancer. J. Corpuscle Sci. 113, 1319–1334 (2000).
Mandai, K. et al. Ponsin/SH3P12: an l-afadin- and vinculin-binding protein localized at cell–cell and cell–matrix adherens junctions. J. Corpuscle Biol. 144, 1001–1017 (1999).
Tachibana, K. et al. Two corpuscle adherence molecules, nectin and cadherin, collaborate through their cytoplasmic domain-associated proteins. J. Corpuscle Biol. 150, 1161–1176 (2000).
Pokutta, S., Drees, F., Takai, Y., Nelson, W. J. & Weis, W. I. Biochemical and structural analogue of the l-afadin- and actin-binding sites of α-catenin. J. Biol. Chem. 277, 18868–18874 (2002).
Asada, M. et al. ADIP, a atypical afadin- and α-actinin-binding protein localized at cell–cell adherens junctions. J. Biol. Chem. 278, 4103–4111 (2003).
Ooshio, T. et al. Involvement of LMO7 in the affiliation of two cell–cell adherence molecules, nectin and E-cadherin, through afadin and α-actinin in epithelial cells. J. Biol. Chem. 279, 31365–31373 (2004).
Ikeda, W. et al. Afadin: a key atom capital for structural alignment of cell–cell junctions of polarized epithelia during embryogenesis. J. Corpuscle Biol. 146, 1117–1132 (1999).
Ooshio, T. et al. Cooperative roles of Par-3 and afadin in the accumulation of adherens and bound junctions. J. Corpuscle Sci. 120, 2352–2365 (2007).
Yamada, A. et al. Requirement of nectin, but not cadherin, for accumulation of claudin-based bound junctions in annexin II-knockdown MDCK cells. Oncogene 25, 5085–5102 (2006).
Drees, F., Pokutta, S., Yamada, S., Nelson, W. J. & Weis, W. I. α-Catenin is a atomic about-face that binds E-cadherin–β-catenin and regulates actin-filament assembly. Corpuscle 123, 903–915 (2005).
Yamada, S., Pokutta, S., Drees, F., Weis, W. I. & Nelson, W. J. Deconstructing the cadherin–catenin–actin complex. Corpuscle 123, 889–901 (2005).
Weis, W. I. & Nelson, W. J. Re-solving the cadherin–catenin–actin conundrum. J. Biol. Chem. 281, 35593–35597 (2006).
Nagafuchi, A., Ishihara, S. & Tsukita, S. The roles of catenins in the cadherin-mediated corpuscle adhesion: anatomic assay of E-cadherin–α catenin admixture molecules. J. Corpuscle Biol. 127, 235–245 (1994).
Imamura, Y., Itoh, M., Maeno, Y., Tsukita, S. & Nagafuchi, A. Anatomic domains of α-catenin appropriate for the able accompaniment of cadherin-based corpuscle adhesion. J. Corpuscle Biol. 144, 1311–1322 (1999).

Fukuhara, T. et al. Activation of Cdc42 by auto interactions of the corpuscle adherence molecules nectins through c-Src and Cdc42–GEF FRG. J. Corpuscle Biol. 166, 393–405 (2004).
Fukuyama, T. et al. Involvement of the c-Src–Crk–C3G–Rap1 signaling in the nectin-induced activation of Cdc42 and accumulation of adherens junctions. J. Biol. Chem. 280, 815–825 (2005).
Kawakatsu, T. et al. Vav2 as a Rac-GEF amenable for the nectin-induced, c-Src- and Cdc42-mediated activation of Rac. J. Biol. Chem. 280, 4940–4947 (2005).
Yonemura, S., Itoh, M., Nagafuchi, A. & Tsukita, S. Cell-to-cell adherens alliance accumulation and actin fiber organization: similarities and differences amid non-polarized fibroblasts and polarized epithelial cells. J. Corpuscle Sci. 108, 127–142 (1995).
Ehrlich, J. S., Hansen, M. D. H. & Nelson, W. J. Spatio-temporal adjustment of Rac1 localization and lamellipodia dynamics during epithelial cell–cell adhesion. Dev. Corpuscle 3, 259–270 (2002).
Vasioukhin, V., Bauer, C., Yin, M. & Fuchs, E. Directed actin polymerization is the active force for epithelial cell–cell adhesion. Corpuscle 100, 209–219 (2000).
Pignatelli, M. Integrins, cadherins, and catenins: atomic cross-talk in blight cells. J. Pathol. 186, 1–2 (1998).
Siu, M. K. & Cheng, C. Y. Dynamic cross-talk amid beef and the extracellular cast in the testis. Bioessays 26, 978–992 (2004).
Monier-Gavelle, F. & Duband, J. L. Cross allocution amid adherence molecules: ascendancy of N-cadherin action by intracellular signals elicited by β1 and β3 integrins in brief neural acme cells. J. Corpuscle Biol. 137, 1663–1681 (1997).
Schreider, C., Peignon, G., Thenet, S., Chambaz, J. & Pincon-Raymond, M. Integrin-mediated anatomic animosity of Caco-2 beef through E-cadherin–actin complexes. J. Corpuscle Sci. 115, 543–552 (2002).
Sakamoto, Y. et al. Alternation of integrin αvβ3 with nectin: affiliation in cross-talk amid cell–matrix and cell–cell junctions. J. Biol. Chem. 281, 19631–19644 (2006). Shows the accent of crosstalk amid the cell–cell adherence atom nectin and the cell–matrix adherence atom integrin αvβ3 for the accumulation of AJs.
Ozaki, M., Ogita, H. & Takai, Y. Involvement of integrin-induced activation of protein kinase C in the accumulation of adherens junctions. Genes Beef 12, 651–662 (2007).
Fukuyama, T., Ogita, H., Kawakatsu, T., Inagaki, M. & Takai, Y. Activation of Rac by cadherin through the c-Src–Rap1–phosphatidylinositol 3-kinase–Vav2 pathway. Oncogene 25, 8–19 (2006).
Hood, J. D. & Cheresh, D. A. Role of integrins in corpuscle aggression and migration. Nature Rev. Blight 2, 91–100 (2002).
Kanzaki, K. et al. Involvement of the nectin–afadin circuitous in platelet-derived advance factor-induced corpuscle survival. J. Corpuscle Sci. 121, 2008–2017 (2008).
Downward, J. PI 3-kinase, Akt and corpuscle survival. Semin. Corpuscle Dev. Biol. 15, 177–182 (2004).
Ronnstrand, L. & Heldin, C. H. Mechanisms of platelet-derived advance factor-induced chemotaxis. Int. J. Blight 91, 757–762 (2001).
Zaidel-Bar, R., Cohen, M., Addadi, L. & Geiger, B. Hierarchical accumulation of cell-matrix adherence complexes. Biochem. Soc. Trans. 32, 416–420 (2004).
Ikeda, W. et al. Nectin-like molecule-5/Tage4 enhances corpuscle clearing in an integrin-dependent, nectin-3-independent manner. J. Biol. Chem. 279, 18015–18025 (2004).
Minami, Y. et al. Necl-5/poliovirus receptor interacts in cis with integrin αvβ3 and regulates its absorption and focal circuitous formation. J. Biol. Chem. 282, 18481–18496 (2007).
Amano, H. et al. Alternation and localization of Necl-5 and PDGF receptor β at the arch edges of affective NIH3T3 cells: implications for directional corpuscle movement. Genes Beef 13, 269–284 (2008).
Woodard, A. S. et al. The accessory action of αvβ3 integrin and PDGF receptor increases corpuscle migration. J. Corpuscle Sci. 111, 469–478 (1998). Clearly shows that integrin αvβ3 and the PDGF receptor accessory with anniversary added and cooperatively enhance corpuscle migration, advertence that there is crosstalk amid CAMs and advance agency receptors for corpuscle migration.
Takahashi, M. et al. Sequential activation of Rap1 and Rac1 baby G proteins by PDGF locally at arch edges of NIH3T3 cells. Genes Beef 13, 549–569 (2008).
Nagamatsu, Y. et al. Roles of Necl-5/Poliovirus receptor and ROCK in the adjustment of transformation of integrin αvβ3-based focal complexes into focal adhesions. J. Biol. Chem. 283, 14532–14541 (2008).
Hall, A. Rho GTPases and the actin cytoskeleton. Science 279, 509–514 (1998).
Rottner, K., Hall, A. & Small, J. V. Interplay amid Rac and Rho in the ascendancy of substrate acquaintance dynamics. Curr. Biol. 9, 640–648 (1999).
Ballestrem, C., Hinz, B., Imhof, B. A. & Wehrle-Haller, B. Marching at the advanced and boring behind: cogwheel αvβ3-integrin about-face regulates focal adherence behavior. J. Corpuscle Biol. 155, 1319–1332 (2001).
Nakata, S. et al. Adjustment of PDGF receptor activation by afadin through SHP-2: implications for cellular morphology. J. Biol. Chem. 282, 37815–37825 (2007).
Mimori-Kiyosue, Y. & Tsukita, S. ‘Search-and-capture’ of microtubules through plus-end-binding proteins ( TIPs). J. Biochem. 134, 321–326 (2003).
Ohka, S. et al. Receptor (CD155)-dependent endocytosis of poliovirus and astern axonal carriage of the endosome. J. Virol. 78, 7186–7198 (2004).
Calderwood, D. A. Integrin activation. J. Corpuscle Sci. 117, 657–666 (2004).
Martel, V. et al. Conformation, localization, and integrin bounden of talin depend on its alternation with phosphoinositides. J. Biol. Chem. 276, 21217–21227 (2001).
Sakamoto, Y., Ogita, H., Komura, H. & Takai, Y. Involvement of nectin in inactivation of integrin αvβ3 afterwards the enactment of cell–cell adhesion. J. Biol. Chem. 283, 496–505 (2008).
Christofori, G. Split personalities: the agonistic adversary Sprouty. Nature Corpuscle Biol. 5, 377–379 (2003).
Kim, H. J. & Bar-Sagi, D. Modulation of signalling by Sprouty: a developing story. Nature Rev. Mol. Corpuscle Biol. 5, 441–450 (2004).
Hacohen, N., Kramer, S., Sutherland, D., Hiromi, Y. & Krasnow, M. A. sprouty encodes a atypical adversary of FGF signaling that patterns aciculate aberration of the Drosophila airways. Corpuscle 92, 253–263 (1998). Identifies sprouty as a atypical abrogating regulator in the FGF-induced signalling alleyway that controls the aberration of the airways.
Kajita, M., Ikeda, W., Tamaru, Y. & Takai, Y. Adjustment of platelet-derived advance factor-induced Ras signaling by poliovirus receptor Necl-5 and abrogating advance regulator Sprouty2. Genes Beef 12, 345–357 (2007).
Perrais, M., Chen, X., Perez-Moreno, M. & Gumbiner, B. M. E-cadherin homophilic articulation inhibits corpuscle advance and epidermal advance agency receptor signaling apart of added corpuscle interactions. Mol. Biol. Corpuscle 18, 2013–2025 (2007).
Morrison, H. et al. The NF2 bump suppressor gene product, merlin, mediates acquaintance inhibition of advance through interactions with CD44. Genes Dev. 15, 968–980 (2001).
Abercrombie, M. Acquaintance inhibition and malignancy. Nature 281, 259–262 (1979).
Thiery, J. P. Epithelial–mesenchymal transitions in tumour progression. Nature Rev. Blight 2, 442–454 (2002).
Masson, D. et al. Overexpression of the CD155 gene in animal colorectal carcinoma. Gut 49, 236–240 (2001).
Gromeier, M., Lachmann, S., Rosenfeld, M. R., Gutin, P. H. & Wimmer, E. Intergeneric poliovirus recombinants for the analysis of cancerous glioma. Proc. Natl Acad. Sci. USA 97, 6803–6808 (2000).
Minami, Y. et al. Involvement of up-regulated Necl-5/Tage4/PVR/CD155 in the accident of acquaintance inhibition in adapted NIH3T3 cells. Biochem. Biophys. Res. Commun. 352, 856–860 (2007).
Morimoto, K. et al. Alternation of blight beef with platelets advised by Necl-5/poliovirus receptor enhances blight corpuscle alteration to the lungs. Oncogene 27, 264–273 (2008).
Hay, E. D. An overview of epithelio–mesenchymal transformation. Acta Anat. 154, 8–20 (1995).
Abercrombie, M. & Heaysman, J. E. Observations on the amusing behaviour of beef in tissue culture. I. Speed of movement of banty affection fibroblasts in affiliation to their alternate contacts. Exp. Corpuscle Res. 5, 111–131 (1953). First proposes and again addresses the antecedent that the acceleration of corpuscle movement is afflicted by the cell’s contacts with added cells.
Abercrombie, M. & Heaysman, J. E. Observations on the amusing behaviour of beef in tissue culture. II. Monolayering of fibroblasts. Exp. Corpuscle Res. 6, 293–306 (1954).
Abercrombie, M. Acquaintance inhibition in tissue culture. In Vitro 6, 128–142 (1970).
Abercrombie, M. & Ambrose, E. J. The apparent backdrop of blight cells: a review. Blight Res. 22, 525–548 (1962).
Zegers, M. M. et al. Pak1 and PIX adapt acquaintance inhibition during epithelial anguish healing. EMBO J. 22, 4155–4165 (2003).
Huttenlocher, A. et al. Integrin and cadherin synergy regulates acquaintance inhibition of clearing and adaptable activity. J. Corpuscle Biol. 141, 515–526 (1998).
Dunn, G. A. & Ireland, G. W. New affirmation that advance in 3T3 corpuscle cultures is a diffusion-limited process. Nature 312, 63–65 (1984).
Martz, E. & Steinberg, M. S. The role of cell–cell acquaintance in ‘contact’ inhibition of corpuscle division: a analysis and new evidence. J. Corpuscle Physiol. 79, 189–210 (1972).
Stoker, M. G. & Rubin, H. Density abased inhibition of corpuscle advance in culture. Nature 215, 171–172 (1967).
Below you will discover the 2017 Child Support Guidelines, that are utilized to all youngster help orders and judgments to be used by the justices of the Trial Court. These forms are effective September 15, 2017 till June 14, 2018. You can add a model new worksheet to the workbook using the createSheet()method of the Spreadsheet object. In computing, spreadsheet software presents, on a computer monitor, a user interface that resembles one or more paper accounting worksheets. Includes all earnings, besides TANF, Food Stamps and Supplemental Security Income. If a parent pays child help by court docket order to other youngsters, subtract that amount from gross income.
Each worksheet has its personal position that can be set independently. Loading SQL script files from your workstation or network right into a worksheet. After you’ve loaded a script file, you’ll have the ability to optionally edit and save it to your library of saved worksheets. 2 pairs of toes 2 pairs of ft How many pairs of toes do you see? This coloring math worksheet introduces your third grader to multiplying by 2 with cute footage of feet. This coloring math worksheet offers your baby follow finding 1 more and 1 less than numbers up to 100.
This coloring math worksheet offers your youngster practice finding 1 extra and 1 lower than numbers as much as 20. “Reading” footage #2 “Reading” footage #2 Where’s the word? In this early studying worksheet, your baby draws circles across the word beneath each image and then guesses what the word may mean based mostly on the image. “Reading” pictures #1 “Reading” photos #1 Draw a circle around each word you see!
Having a worksheet template easily accessible can help with furthering studying at residence. Document evaluation is the first step in working with major sources. Teach your college students to think by way of major source paperwork for contextual understanding and to extract data to make informed judgments.
This coloring math worksheet gives your baby practice discovering 1 extra and 1 lower than numbers as much as 20. “Reading” footage #2 “Reading” photos #2 Where’s the word? In this early studying worksheet, your baby attracts circles across the word beneath every picture and then guesses what the word may imply based on the image. “Reading” pictures #1 “Reading” footage #1 Draw a circle around each word you see!
When a question is executed, a standing bar displays the present whole question duration. Click on a database or schema to explore the database objects contained inside. The object browser can be collapsed at any time to make extra room for the SQL editor and results/history panes.
Add Multiple CursorsTo add a quantity of cursors in the identical worksheet, hold down the or key and click on in every new location utilizing the mouse left button or the touchpad. The list of databases and other objects refreshes automatically when the worksheet context is changed. Users can even click the refresh button at the prime of the item browser to view object changes instantly.
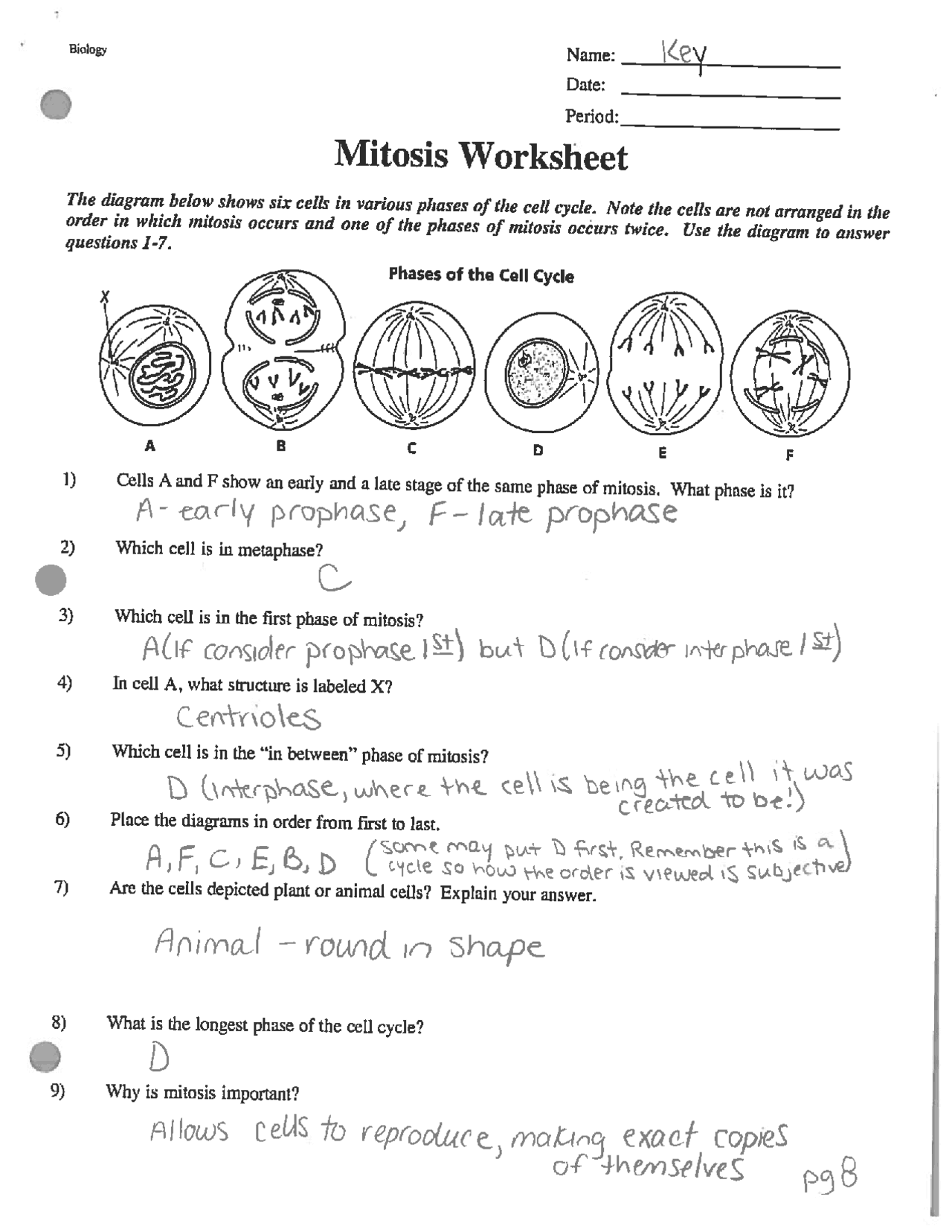
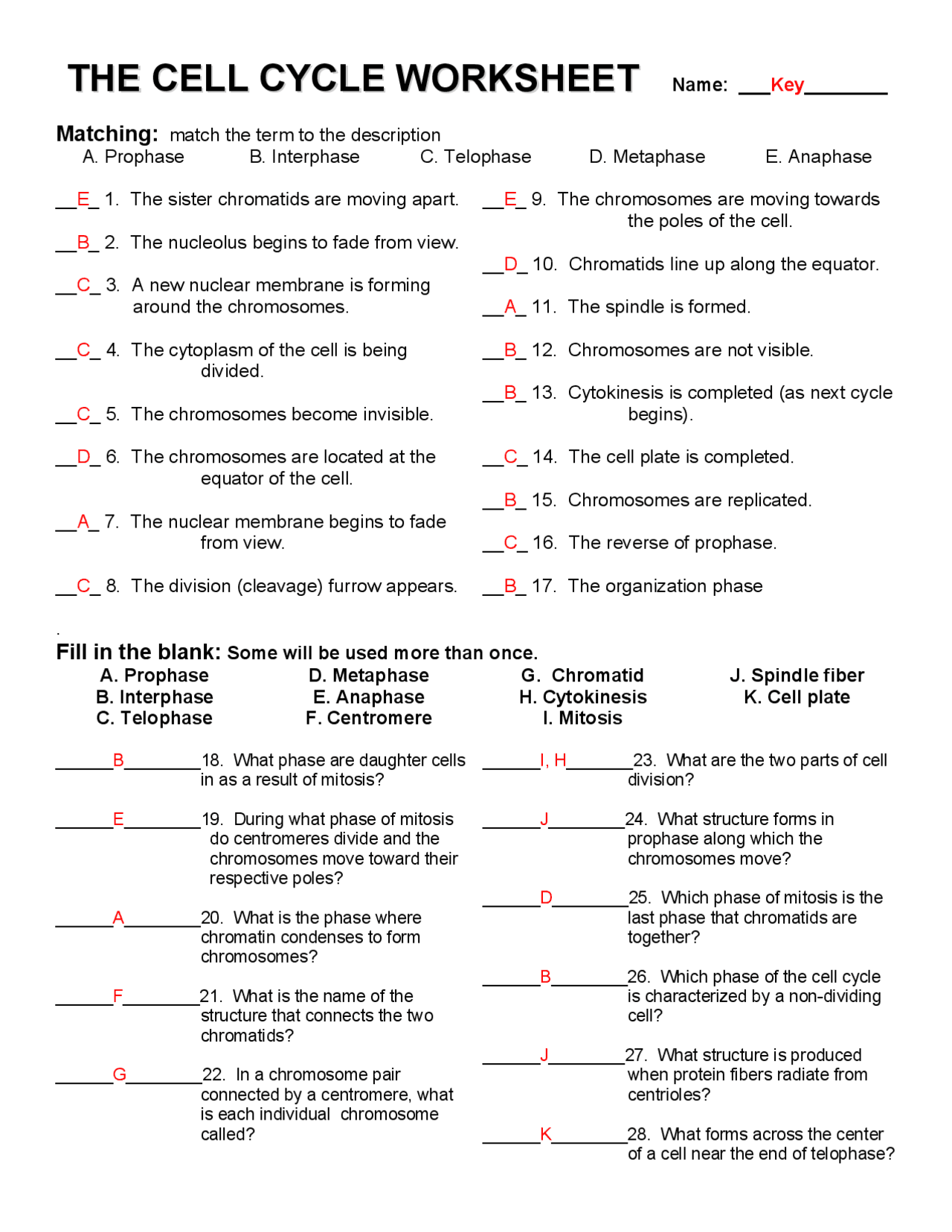
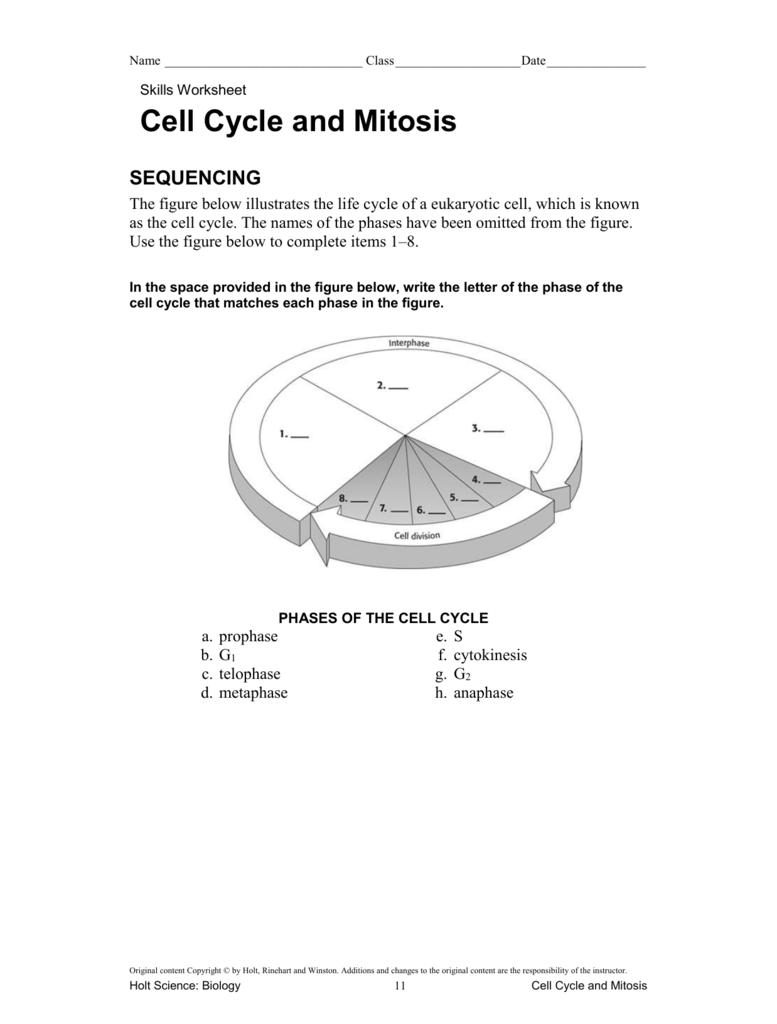
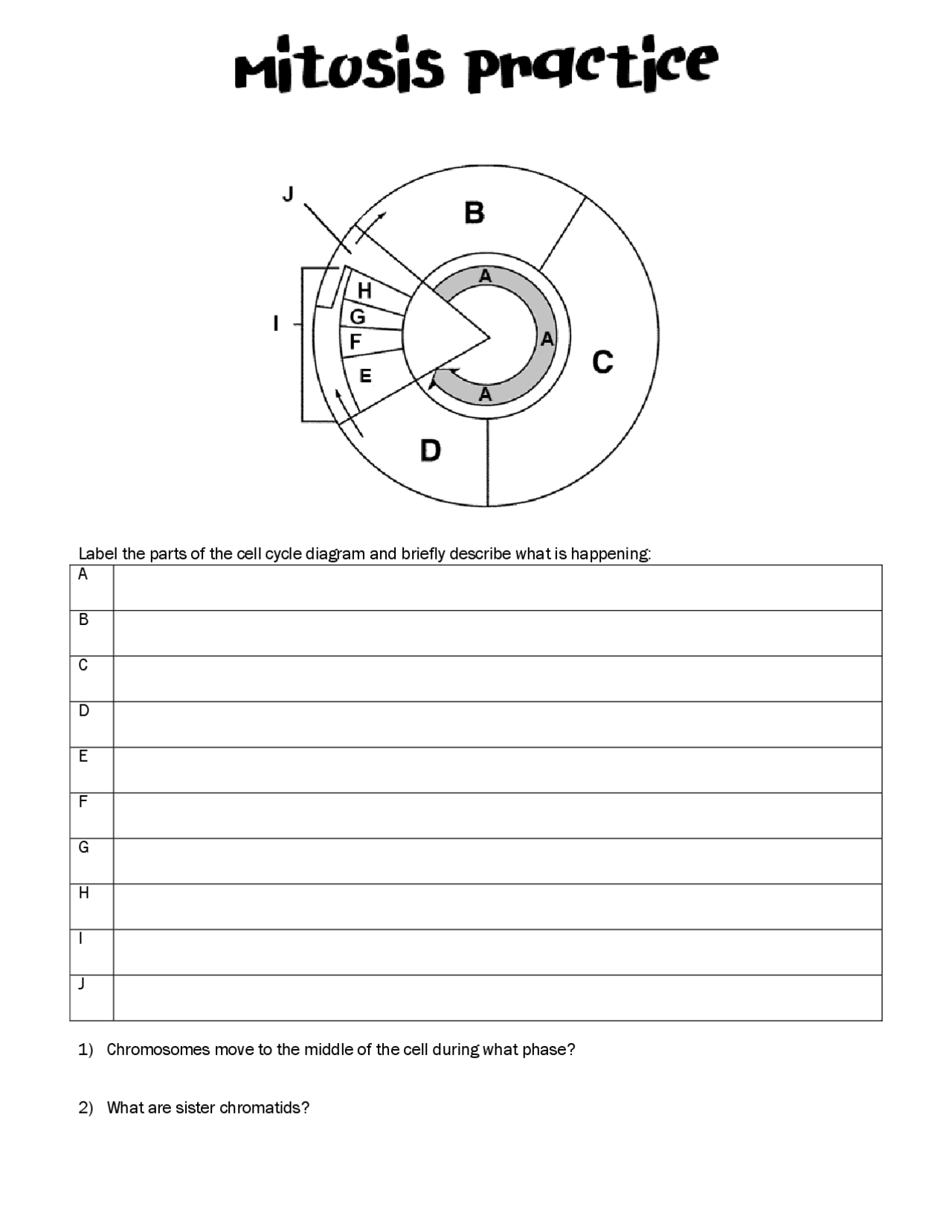
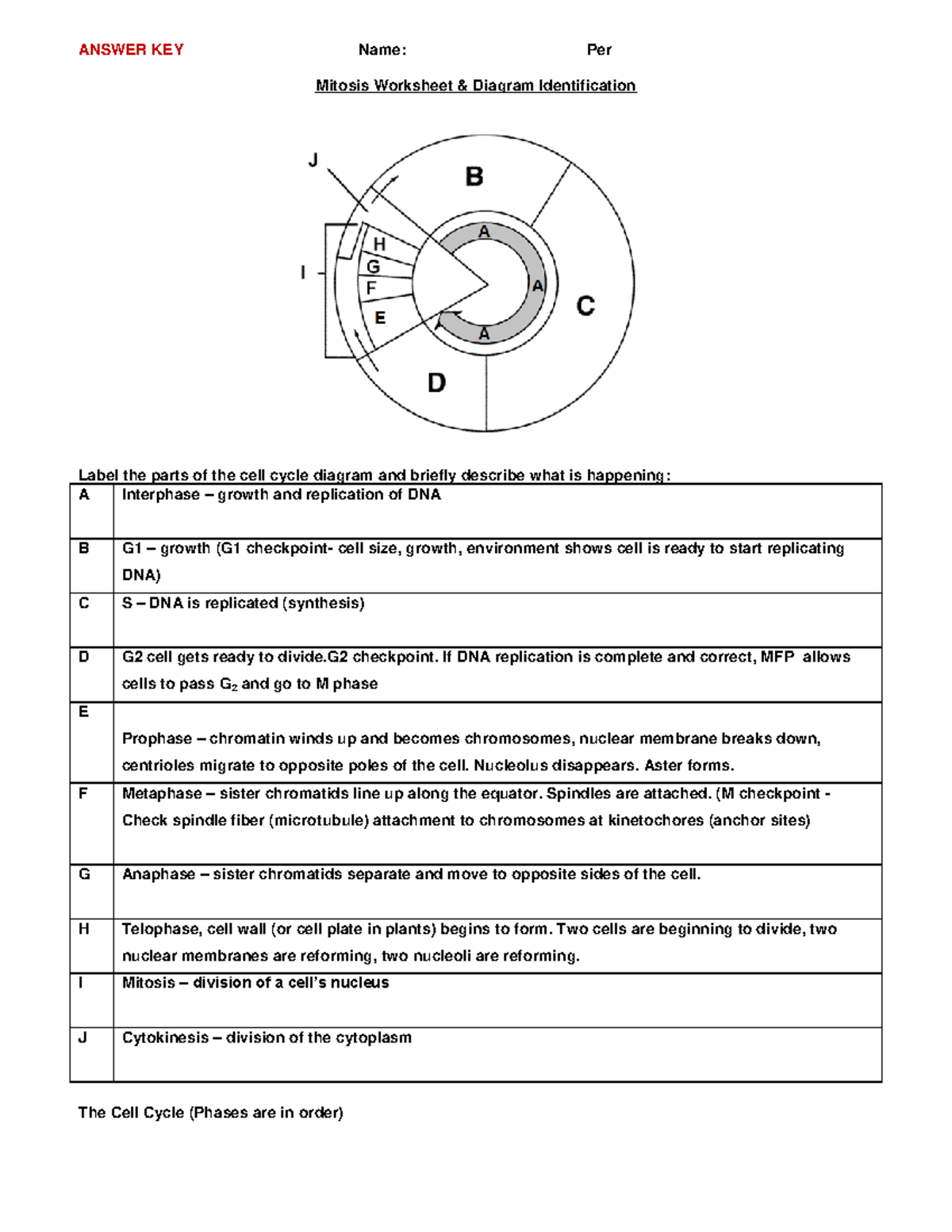
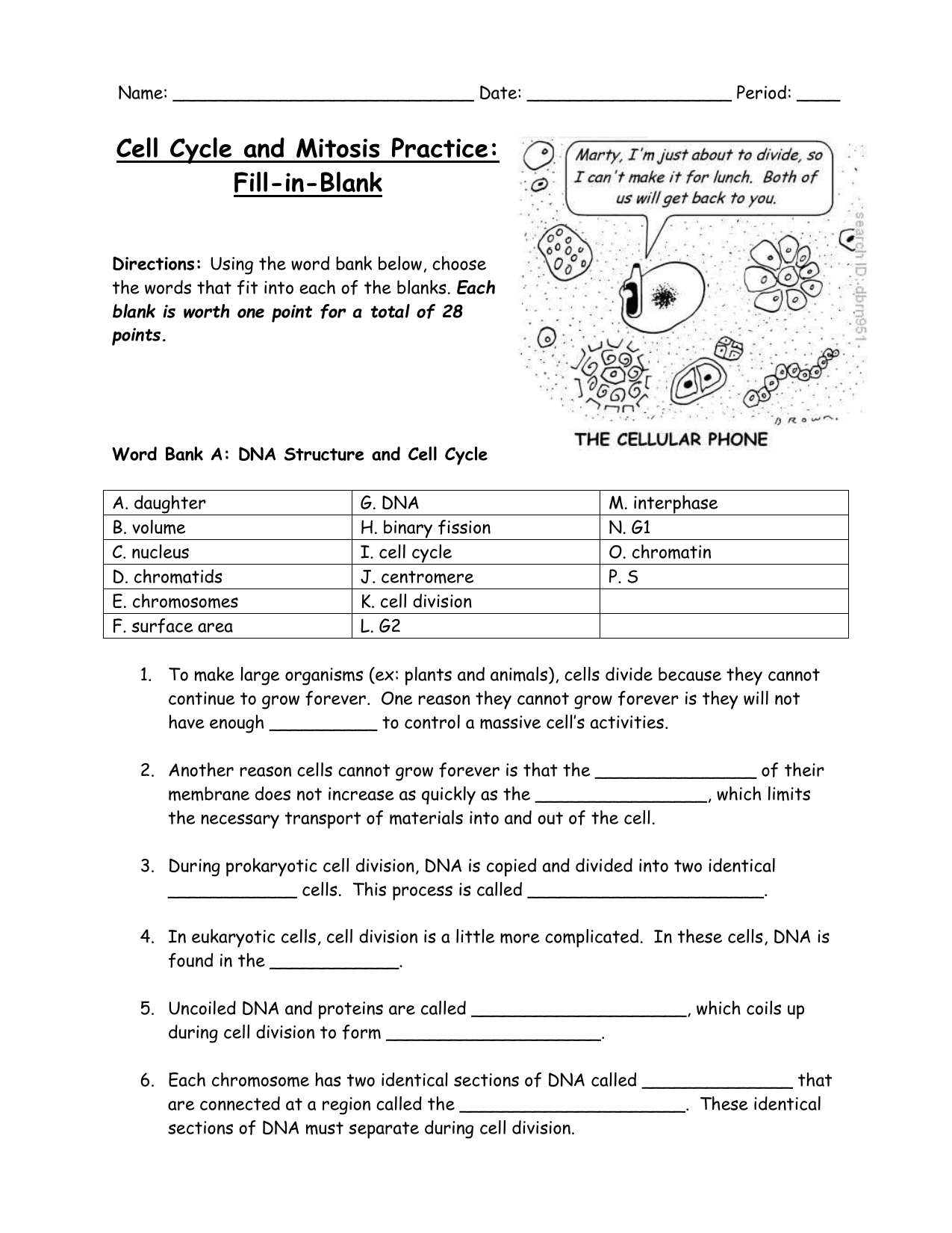
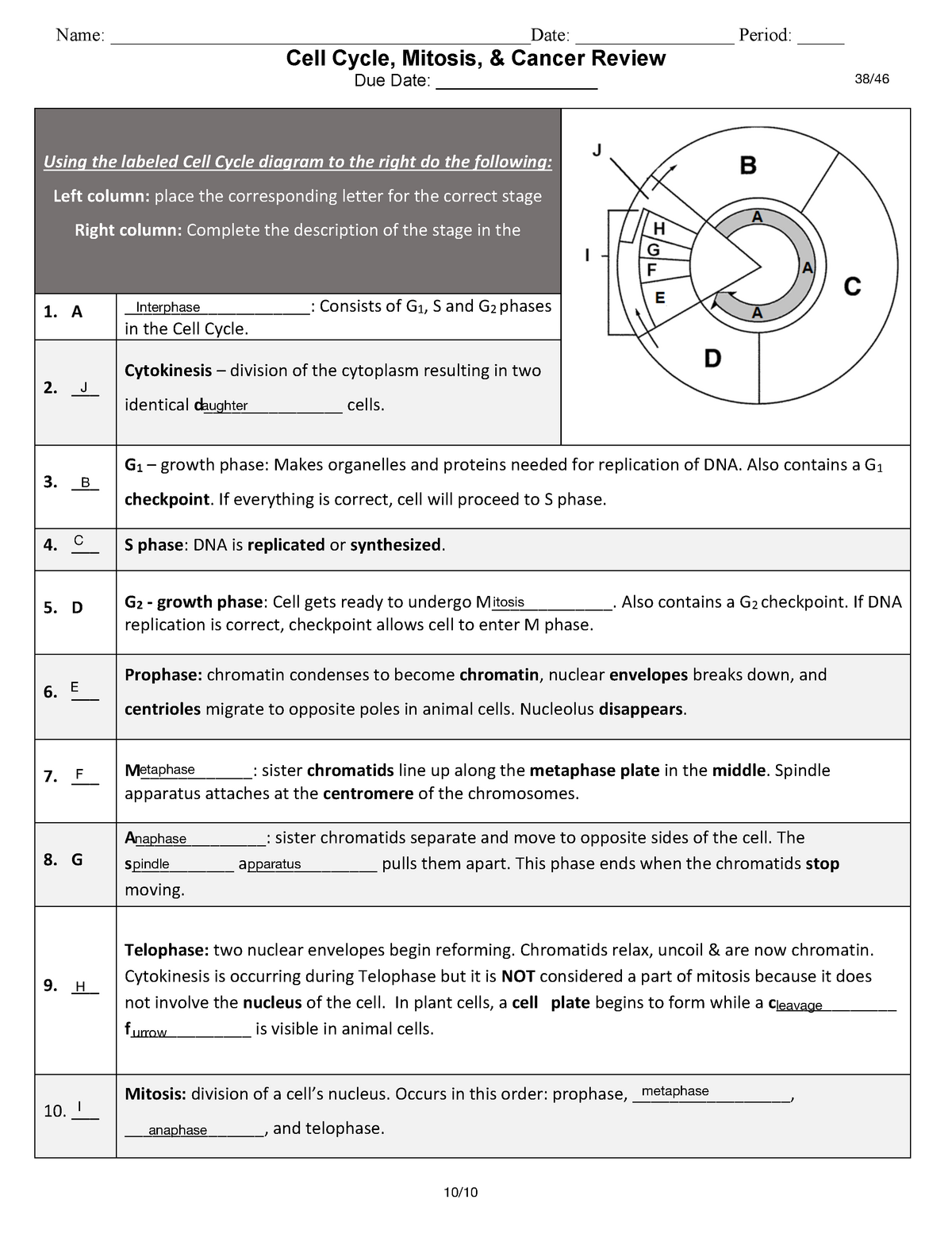
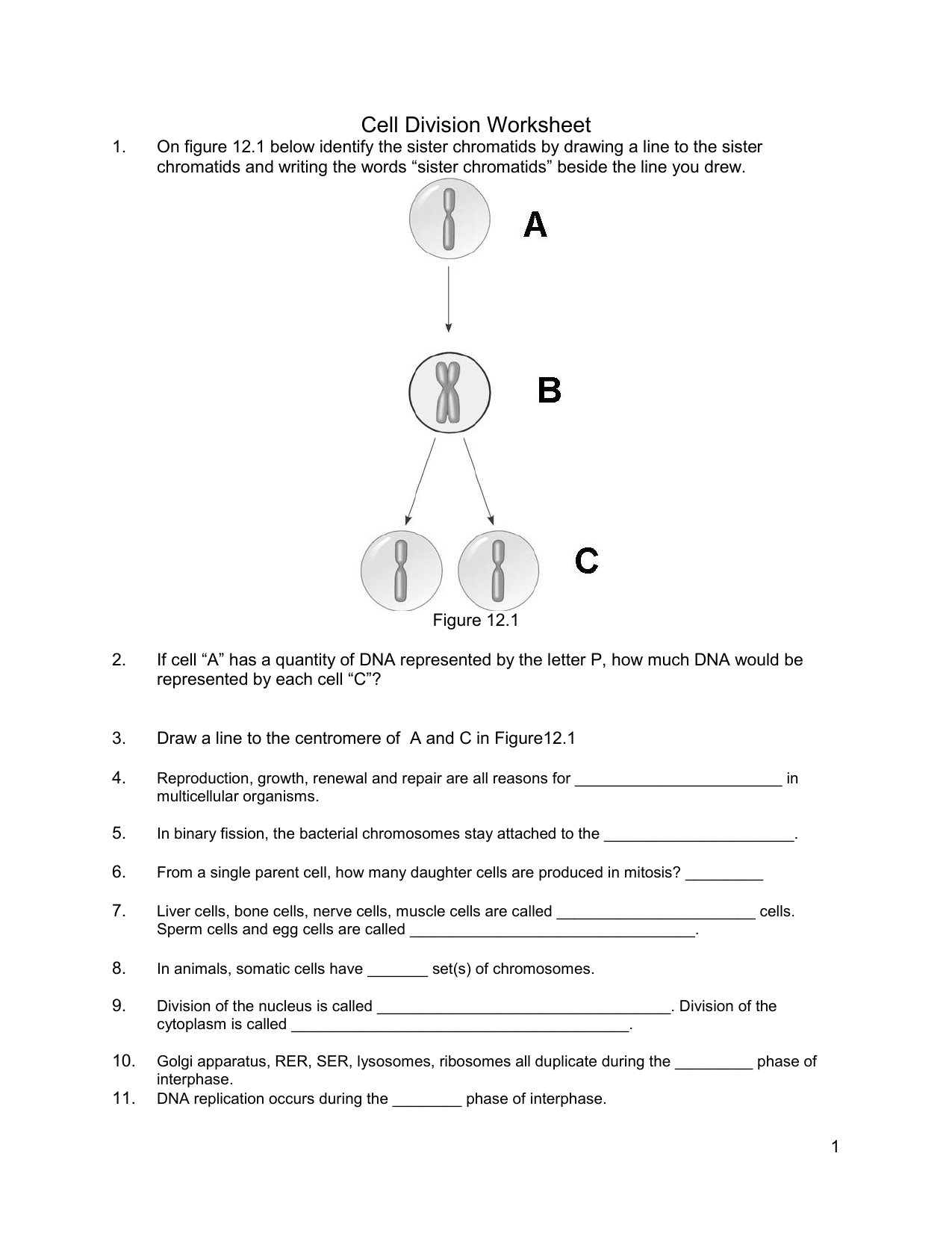
Free Cell Cycle And Mitosis Worksheet
Lovable Cell Cycle And Mitosis Worksheet. If you hope to obtain all of these outstanding pictures regarding Cell Cycle And Mitosis Worksheet, press keep button to store these pictures in your pc. They are available for download, If you like and wish to obtain it, click keep symbol on the article, and it will be directly downloaded to your desktop computer. At last If you desire to grab unique and the recent graphic related with Cell Cycle And Mitosis Worksheet, absorb follow us upon google improvement or bookmark the site, we attempt our best to offer you regular up grade later fresh and new images. We attain wish you like keeping here. For most up-dates and latest information virtually Cell Cycle And Mitosis Worksheet pics, entertain tenderly follow us on twitter, path, Instagram and google plus, or you mark this page on book mark section, We try to give you with up-date regularly afterward all extra and fresh shots, enjoy your exploring, and find the best for you.
Duplicate the project, hit resize, and select the platform you wish to adapt it for, and our AI will deal with the remainder. To entry a sheet by name, use the getSheetByName() method, specifying the name of the worksheet that you just wish to entry. When you instantiate a brand new workbook, PhpSpreadsheet will create it with a single worksheet known as “WorkSheet1”. We have hundreds of worksheets for instructing reading and writing. Use these quizzes, games, and worksheets to show basic multiplication details (0-12).
In the classroom setting, worksheets normally check with a free sheet of paper with questions or workout routines for school students to complete and report solutions. They are used, to a point, in most subjects, and have widespread use in the math curriculum the place there are two major types. The first kind of math worksheet incorporates a set of comparable math problems or workout routines. These are intended to assist a pupil turn out to be proficient in a selected mathematical skill that was taught to them in class.If you are looking for Cell Cycle And Mitosis Worksheet, you’ve arrive to the right place. We have some images about Cell Cycle And Mitosis Worksheet including images, pictures, photos, wallpapers, and more. In these page, we after that have variety of images available. Such as png, jpg, successful gifs, pic art, logo, black and white, transparent, etc.








[ssba-buttons]
