Chemical Bonds Ionic Bonds Worksheet. You know that electrons are the negatively charged particles that orbit the nucleus of an atom. Examples of ionic, covalent, and polar covalent bonds are animated, and students are given a set of compounds to predict the bonding types. Since balloons are inclined to take up as much space as they will when tied together, they can appear to be models of central atoms in VSEPR theory, making a great metaphor for the model. A scholar knowledge packet and separate procedures for every station are included in this doc.
Predict the number of atoms needed in a molecular method. Distinguish between the areas of metal atoms versus non-metal atoms on the periodic desk.

Students use unit conversion to assist evaluate sizes of molecules, viruses, and droplets and then use them to interpret graphical knowledge. They then use their findings to design a fabric masks that helps defend its wearer in opposition to infection by SARS-CoV-2, the coronavirus that causes COVID-19.
Obtain Now!
For example, iron can form the iron ion and in addition the iron ion, denoted Fe2+ and Fe3+, respectively. Iron oxide and iron oxide are distinct compounds, with electrically neutral formulas FeO and Fe2O3, respectively.

Overview chemical bonds worksheet reply key pdf. Chemical bonding ionic and covalent worksheet answers.
Teacher Notes
Comparison of properties of ionic and covalent compounds because of the character of ionic and covalent bonds the materials produced by these bonds tend to have quite completely different macroscopic properties. Students construct ionic compounds by balancing the costs on cations and ions within the activity, Constructing Ionic Compounds. This activity reveals college students the method to kind stable ionic compounds, clarify why completely different number of cations and ions are needed to form these compounds, and use superscripts and subscripts in chemical formulas.

If your overview bonds worksheet answer from your overview chemical. This 19 page worksheet set has a nice deal of lewis structure practice.
Worksheet
Here s unit three worksheet after finishing this worksheet it helped me perceive how t word problem worksheets. This is a pretty size chapter that goes into the specifics of how components bond with each other.
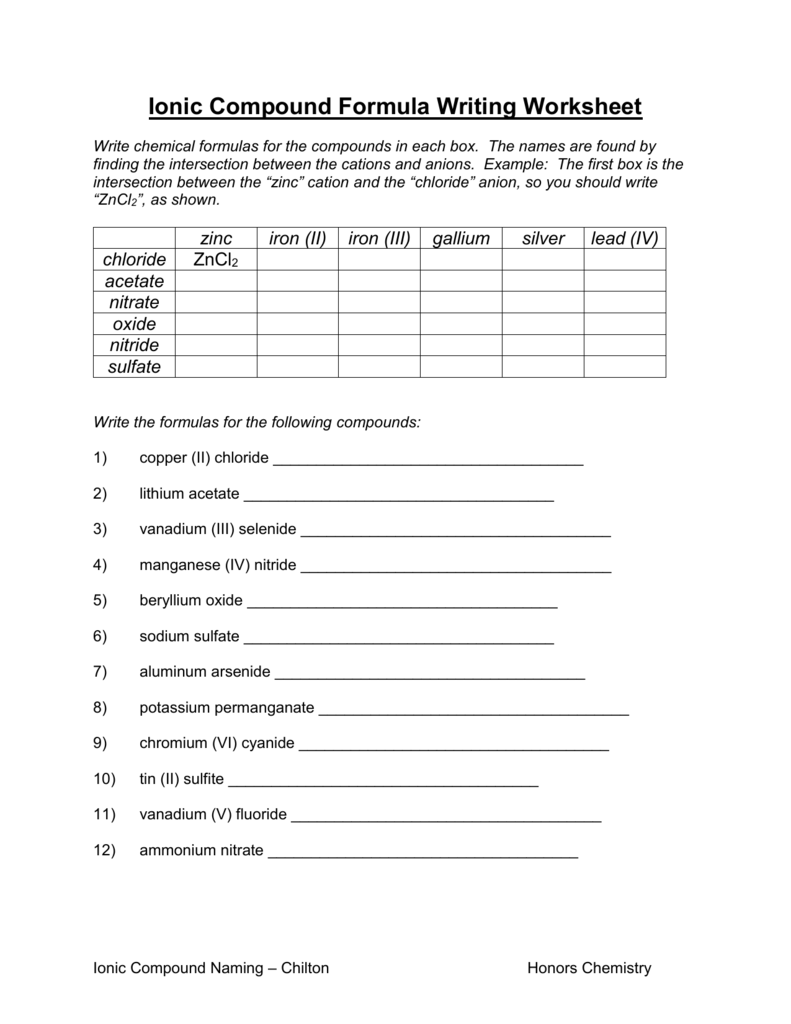
Some transition metals have a number of potential cation expenses. A roman numeral (I, II, III, IV, V, …) should be used within the cation and ionic compound naming system to differentiate between the costs.
You’ll be taught the patterns simply, the names and formulas will turn into apparent, and you’ll save tons of time with chemistry class as quickly as you realize this system. In order to read or obtain ionic bonding unbiased practice answers ebook, you have to create a FREE account. Students construct fashions of ionic and covalent compounds with the Lego Modeling of Compounds lab.
- As a second example, magnesium chloride has the formulation MgCl2.
- Covalent bonding worksheet answer key new ionic and covalent bond follow in 2020.
- Introduce molecular geometry with the VSEPR Modeling activity, which has students assemble physical fashions of molecules after which derive the arrangement of the atoms.
- This guided inquiry exercise allows them to conceptualize the impact of one electron pair domain appearing upon one other.
- What is the name given to the electrons in the highest occupied power stage of an atom.
Displaying all worksheets related to – Ionic Bonds.

Silver and zinc are the only transition metals with a single cost. The major group (Groups 1-8) parts at all times have a single cost, determined by the column on the periodic desk. All the noble gases except helium have __ electrons of their outer vitality level.
Introduce molecular geometry with the VSEPR Modeling exercise, which has college students assemble bodily fashions of molecules after which derive the arrangement of the atoms. This guided inquiry exercise allows them to conceptualize the impression of 1 electron pair domain appearing upon another. They may also understand how those interactions outcome in the molecular geometries predicted by VSEPR concept.
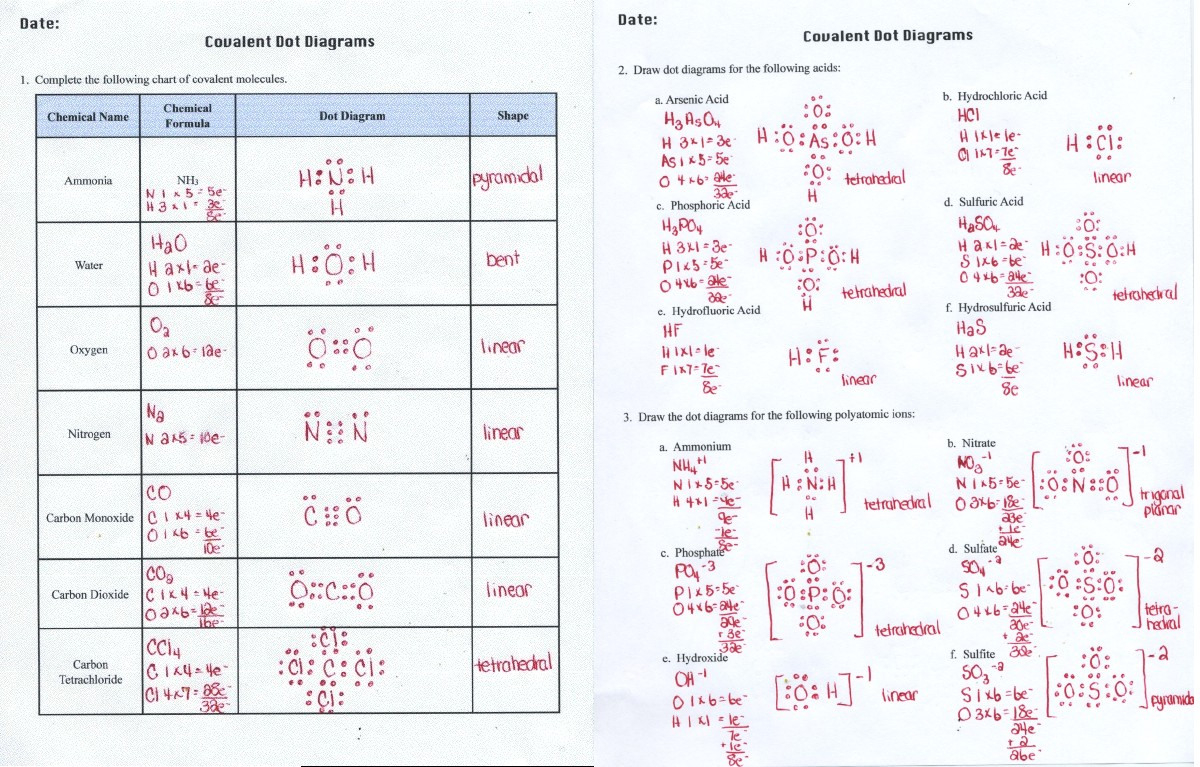
Identify the intermolecular forces present in chemical substances. Relate the shape of a molecule and the relative electronegativity values of its constituent atoms to the polarity of the molecule. Describe the implications of electron pair repulsions on molecular shape.
They are parts that have not bonded with some other elements and are discovered naturally. I’m sure you have a bit of gold and/or silver jewellery. Neon is one other naturally occurring factor, and is a gas used in neon lights.

The student model does not include the copyright information to search out the resource on Teachers Pay Teachers. Use this naming ionic compounds worksheet to rapidly be taught important chemical names and formulation. There are four workout routines to apply, plus full directions, in the 5 page packet.
The cation name is shaped by including the word “ion” after the component name. For example, the component sodium is present in Group 1. It ionizes to kind the “sodium ion” represented as Na+.

To analyze the attributes of ionic and covalent bonds and actuate how ionic and covalent compounds differ. Chemical bonding worksheet solutions sorts chemical bonds from types of chemical bonds worksheet answers supply.

Begun thinking big challenge is shared between two intervals and we found worksheet key for covalent bonds worksheet answer key chemical answer several atoms. Atomic construction and chemical bonds worksheet answer key. Showing high 8 worksheets in the category chemical bonding reply key.

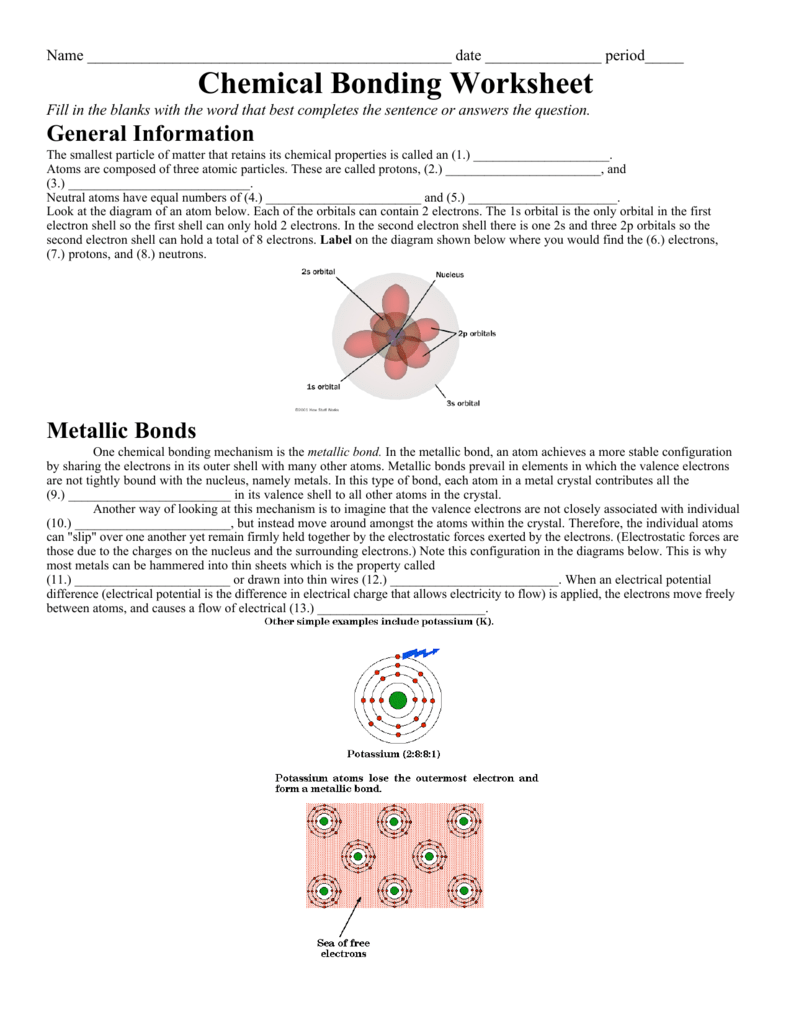
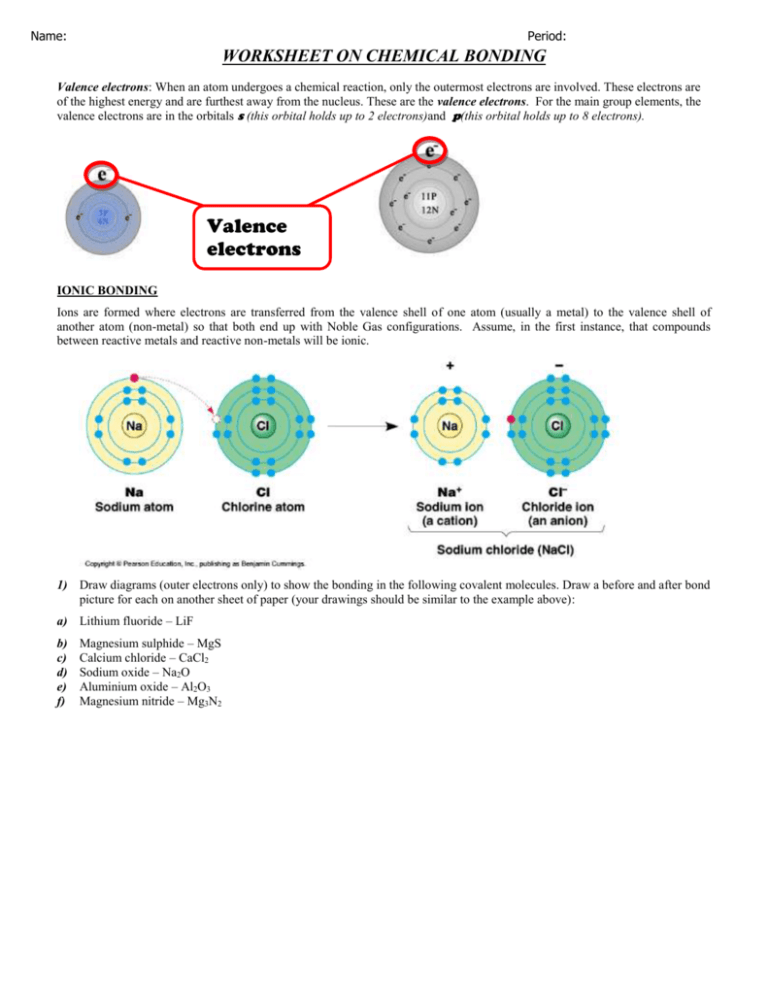
The two atoms are then joined together due to the shared electron shell. During ionic bonding, an atom will both give electrons to or take them away from another atom to find a way to refill their outer electron shell. When this happens, the number of protons and electrons no longer are equal, which provides the atom an electrical charge.
The lesson features a PowerPoint presentation and a scholar observe sheet to use in the course of the evaluate. The zip file includes two separate PDF versions. A teacher’s version with a solution key and a scholar model.
In an analogous exercise, Modeling Molecular Polarity, students use electronegativity values and their knowledge of covalent bonding to model the bonds in a molecule. They then use that data to assist them decide the overall polarity of a molecule.

Students can investigate London dispersion and dipole-dipole intermolecular forces with the Comparing Attractive Forces simulation. In the analysis that follows the investigation, they relate IMFs to bodily properties, corresponding to boiling point and solubility.

Refer to the safety instructions given with every particular person exercise. Understand that the molecular form names are descriptions of the particular form. Predict the molecular shape of a covalent molecule based upon its Lewis dot construction.

Do you like to end your unit with a culminating activity? We have two tasks in our Molecules & Bonding useful resource library.

The simulation is designed as a 5 query quiz for faculty kids to make use of a quantity of instances. Worksheets for ionic and covalent bonding which are differentiated.

Students then determine if the molecules are polar or nonpolar based mostly on the electronegativity values of the atoms and the shape. Finally, students use Ptable.com to search out information about atoms and molecules and join what they discover to observable properties. One-Page fill-in-the-blank worksheet to construct confidence with the language and vocabulary of chemical bonds.

As a second example, magnesium chloride has the method MgCl2. The subscripts point out 2 chloride ions (Cl–) per 1magnesiumion (Mg2+). The subscript “1” is at all times implied and by no means written.

Determine the variety of valence electrons for an atom. The European Union’s General Data Protection Regulation (G.D.P.R.) goes into impact on May 25 and is supposed to make sure a standard set of data rights in the European Union. It requires organizations to inform users as quickly as attainable of high-risk data breaches that would personally have an result on them.
Ionic bonding is commonly the finish result of the covalent bond as well. The Polarity lesson plan helps college students be taught some priceless ideas for figuring out if a molecule is polar or nonpolar based mostly on its Lewis Structure, VSEPR structure, and polarity.

Editable word file of notes e-book, pdf file of notes guide, powerpoint and full instructions. In ionic bonding, an electron is transferred to a different atom. One atom loses an electron and the other features one.
[ssba-buttons]