Electron Configuration Practice Worksheet Answers. Note that there’s a pattern to electron configurations.This video worksheet will assist you to understand the pattern. In chemistry, electron dot configuration has its own significance and this illustration of valence electrons was invented by American chemist Gilbert Newton Lewis. Hence, we can’t predict the number of valence electrons of a transition steel with sure quantity. This really isn’t shocking, given the aufbau precept idea that each element may have the electrons in the same place as the component earlier than it, plus one.
This actually isn’t stunning, given the aufbau principle idea that each element will have the electrons in the identical place because the element before it, plus one. However, it ought to make you sort of marvel why anyone would go to the trouble of writing a bunch of phrases that will all be the identical, anyway. The first two electrons in lithium are in the same place as those in helium.

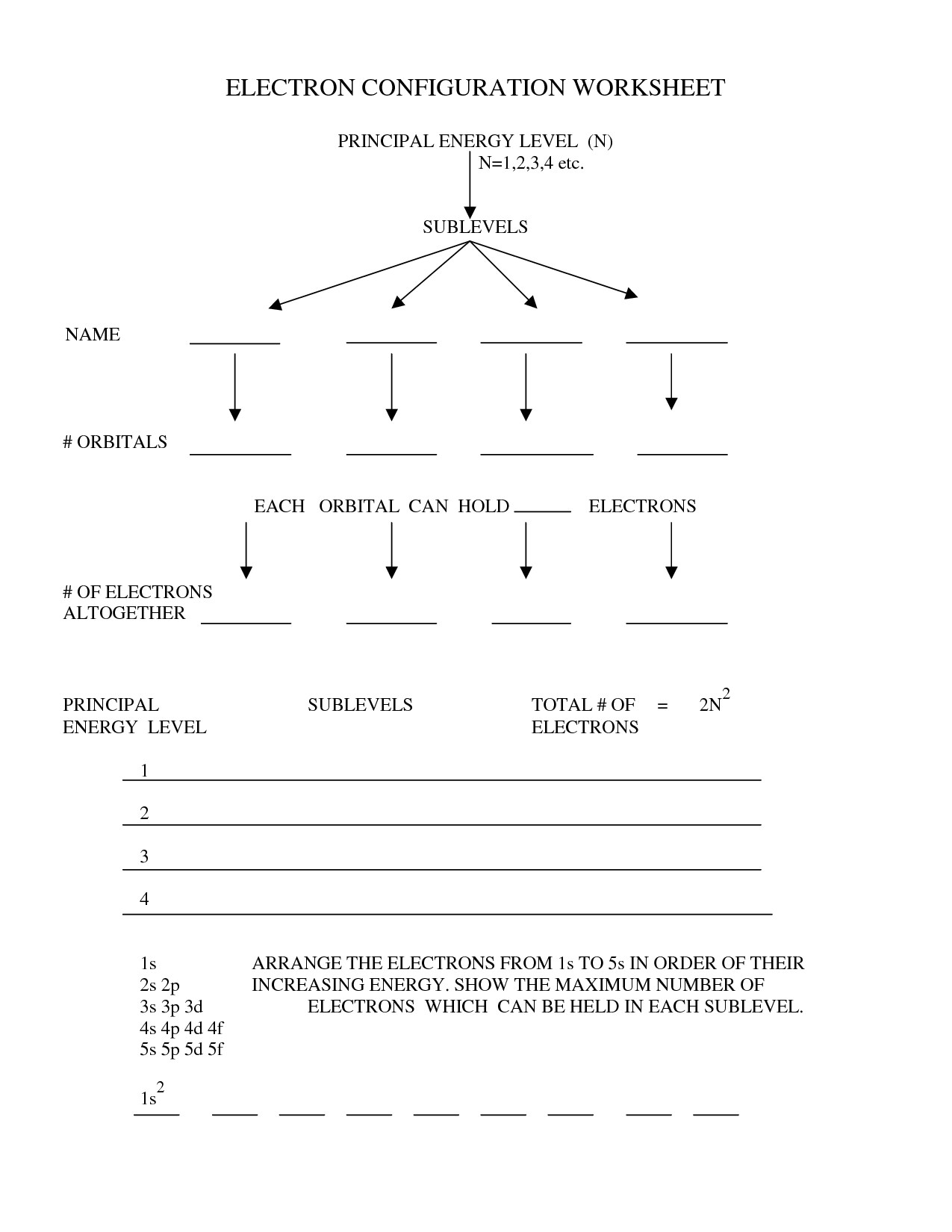
As the name proposes, ‘n’ is the chief power stage where the electron is definitely detectable. And the ‘n’ worth is decided primarily based on the gap of power degree from the nucleus of the atom.
Electron Dot Configuration
These 4 atomic orbitals are current across the nucleus of an atom and represent totally different vitality states. In these instances, the electron configuration is understood to be the same as earlier than, except that the half stands for “the electron configuration of Xe”. Or, in other phrases, it’s written instead of 1s22s22p63s23p64s23d104p65s24d105p6when writing electron configuratons.
- If these terms don’t even look acquainted, you’re either a chemistry scholar who needs plenty of help or are on the wrong web site.
- And the ‘n’ worth is determined based mostly on the distance of vitality stage from the nucleus of the atom.
- These great outlines of geometrical positioning of electrons symbolize completely different states across the nucleus known as atomic orbitals.
- Hund’s rule denotes that electrons must occupy every single orbital of a subshell with a minimum of one electron with same spin course.
- The primary objective of angular quantum quantity is to denote the orbital shape and the sort of subshell of an electron occupies.
Rather than having me discuss this at nice length, it’s probably higher that I just do a bunch of examples and clarify what I’m doing as I follow along. This will appear frustrating at first, but sooner or later you’ll simply understand it and be carried out with the whole thing.
Definition And Basics Of Digital Configuration
1) Pick a periodic table the place you can see all of the variety of components within the boxes. And learn about periodic desk terminology like rows, columns, periods and teams. However, Hund’s rule strictly follows the speculation of atomic spectra.
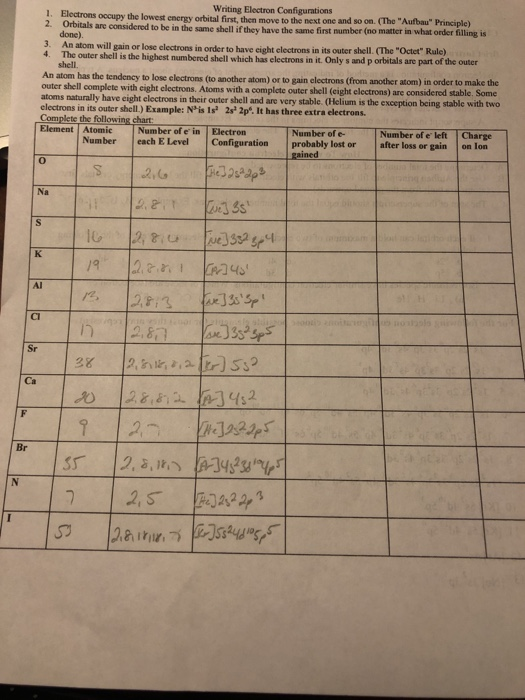
Atoms become ions after they lose or achieve electrons. It helps us to grasp the reactivity of varied components. It turns out that electron configurations give us info similar to how many bonds something will wish to make, as nicely as what number of electrons something needs.

It consists of 40 electrons in whole in the shells. 7) If essential, you’ll be able to transform the lone pair of electrons into bond pair of electrons to fulfil octet rule.
Most Value Of Laptop Depend Hackerrank Resolution In C
As a outcome, we start off with the time period 1s² to explain their location. You can even see that when you count from the top of the periodic table to lithium, you need to rely two areas over within the 1s section to get to lithium. Now, you might recognize this as being totally different than your normal periodic desk.
4) The least potential electronegative atom or ion is placed in the midst of the molecule and join the atoms utilizing single bonds solely. 2) In case of anion molecule, add the extra electrons across the element while drawing dot diagram.
Electron Configurations
An electron configuration is a list that reveals you where all of the electrons in an atom are located. It simply tells you the place the electrons are.
Electron configuration apply worksheet answer key chemistry. The apply is a crucial factor in grasp any skill. You need the hours you place to be the best.
Write a set of quantum numbers for every of the electrons with an n of three in a Sc atom. Barium is a highly reactive alkaline earth steel with atomic number 56 and bears the symbol ‘Ba’. Since it’s highly reactive, we cannot discover this steel in its free state and at all times stays in combination with other metals.

An investigation confirmed the trigger to be the absence of adequate cobalt in the soil. Cobalt forms cations in two oxidation states, Co2+ and Co3+.

Download best free printable electron configuration worksheets with answers. Fill Electron Configuration Worksheet Answer Key W311, Edit online. Sign, fax and printable from PC, iPad, tablet or mobile with pdfFiller Instantly.

And hence the electronic configuration of bromine atom is 1s22s22p63s23p64s23d104p5, satisfying Aufbau precept. And whereas replacing the noble gas component is written in sq. brackets.
These values vary start from 1 to n…, while n denotes the worth of the outermost shell occupied with electron. As we all already know, electrons bear cost i.e. either negative or constructive, and are free to vary their locations often. Their movement from one power state to a different utterly is determined by the engaging and repulsive forces between the positive and unfavorable expenses.

Hence, unabbreviated electron configuration stays much longer, confused and time-taking. Carbon atom consists of no lone pair of electrons since it has four valence electrons.
The digit on the ones place of the group number refers to the variety of valence electrons of an element. To put it merely, each particular person electron encompasses of four quantum numbers and two electrons should exhibit reverse spins when positioned in the same orbital.

The unabbreviated form of electron configuration is the configuration that doesn’t utilise noble gasoline notation whereas writing the electron configuration of elements. Let us learn extra in regards to the electronic configuration along with some superior worksheets and orbital diagrams on this article.

Whereas orbital diagram is an illustrative representation of location and spin of the electrons inside the orbitals within the form of arrows. Read Book Electron Configuration Worksheet Answer Electron Configuration Worksheet Answer Thank you very a lot for reading electron configuration worksheet answer. Interactive worksheets maker – generador de fichas interactivas.

To differentiate the weather into different blocks and teams such as s-block, p-block, d-block and f-block elements. To find out components that show similar chemical and bodily properties. The carbon atom is the central atom of the molecule.

But writing electronic configuration of elements within the periodic desk that come after noble gasoline group is lengthy and tedious. View Homework Help – And electron configuration practice worksheet from CHEMISTRY one hundred and one at Miami Beach Senior High School.
2) Choose any element of your selection from the periodic table. What is Hund’s rule of most multiplicity? And that’s the one difference between the d-orbitals and the other ones.

U 1s2 2s2 2p6 3s2 3p6 4s2 3d104p6 5s2 4d10 5p6 6s2. If these terms are complicated you, you’re in the proper place. If these phrases don’t even look familiar, you’re both a chemistry student who needs a lot of help or are on the mistaken web site.

SolutionAlthough both and are correct, encompasses both and is the most effective answer. 6) Check out for each atom whether or not it possess octet configuration. If any atom does not have octet configuration, then you should fulfil the octet valence of each individual atom.

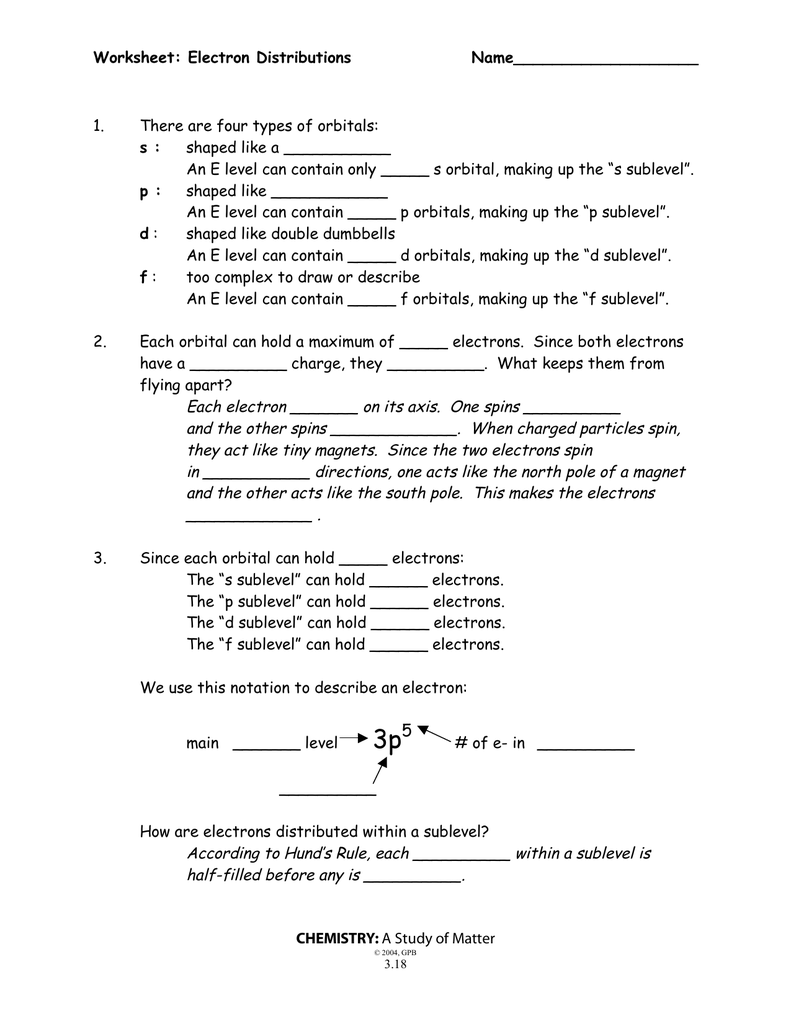
The extra electron configuration follow issues you do the higher you will. S, P, D and F are the four completely different atomic orbitals located across the nucleus of an atom with completely different power ranges.

The ground state electron configuration of Fe is _____. The ground state electron configuration of Ga is _____.

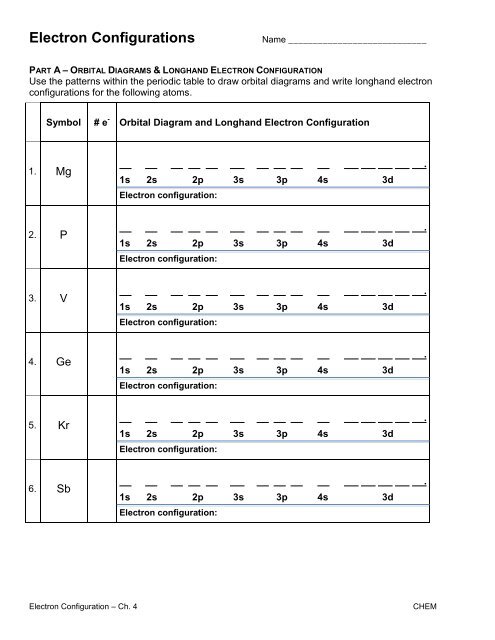
Symbol # e- Longhand Electron Configuration Notation 7. Pb 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p2 9.

According to the principles of electronic configuration, two electrons can find in the identical orbital but with opposite spin directions. If the worth of ms is +1/2 for an electron, then that electron is ‘alpha electron’ while the electron with -1/2 spin value is ‘beta electron’. These wonderful outlines of geometrical positioning of electrons represent completely different states around the nucleus referred to as atomic orbitals.

Hund’s rule denotes that electrons should occupy each single orbital of a subshell with at least one electron with identical spin path. And then they will start double occupying of orbitals of subshell. To decide the electronic configuration of an element, one should follow three essential ideas from quantum mechanics.
[ssba-buttons]